Orthosilicates
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Orthosilicates
These are orthosilicates; the comma between the Mg and the Fe means that these metals can replace each other in any proportions. Xenolite, it will be observed, is the silicate of a triad metal, aluminium; and four atoms of aluminium replace twelve molecules of hydrogen in three molecules of orthosilicic acid. But double silicates are common, in which three of the atoms of hydrogen in three molecules of orthosilicic acid may be replaced by three monad atoms; or by one dyad and one monad atom ; or we may have a monad group, such as -A1=0, or - A1F2, replacing each atom of hydrogen ; or, lastly, the aluminium may be partly hydroxide, thus constituting a basic silicate. Examples of such compounds are:-
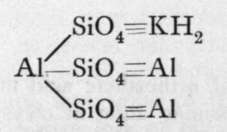
Muscovite, or Potash mica.
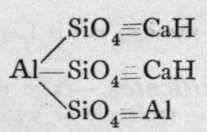
Prehnite.
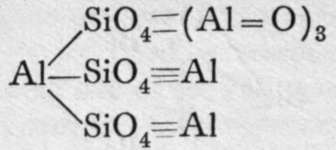
Fibrolite.
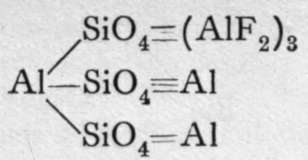
Topaz.
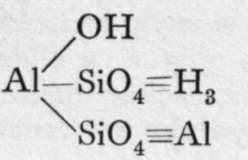
Kaolin or China-clay.
In such silicates, the aluminium is often partially replaced by triad metals, such as triad chromium, iron, or manganese.
Continue to:
- prev: Osmosis
- Table of Contents
- next: Metasilicates
