Osmosis
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Osmosis
To separate the ions of sodium chloride and of hydrochloric acid advantage is taken of a discovery made by Graham, that a vegetable or animal membrane like parchment or parchmentised paper is readily permeated by crystalline bodies, while it is very slowly permeated by " colloidal" or gum-like compounds. By placing in a drum, floating on water, the mixture of orthosilicic acid and salt, the sodium and chlorine ions pass through, of course in equivalent proportions, leaving the colloid behind. Fresh water is substituted from time to time, until all chlorine ions have been removed from the silicic acid. The water can be removed by evaporation in vacuo, and a clear but very viscous liquid remains, which is believed to contain Si(OH)4.Aq. On raising the temperature of this viscous liquid it gelatinises, and is then insoluble in water ; the resulting compound may have a formula analogous to carbonic acid, 0=Si(OH)2; it is termed metasilicic acid. On further drying, water is gradually expelled, and finally a flint-like mass is left, which on ignition yields a white powder of SiO2, or silica. As already mentioned, silica is found in nature; when pure, it crystallises in hexagonal prisms, and is termed quartz, rock-crystal, or Irish diamond. It is used tor spectacle lenses and optical instruments.
The major part of the rocks which constitute the surface of the earth consists of mixtures of silicates. Occasionally they are found in definite crystals, and on analysis their formulae can be determined. From their formulae, the formulae of the silicic acids from which they may be supposed to be derived can be deduced; and tables follow, in which the formulae of these silicic acids and of some of the minerals constituting their salts are given.
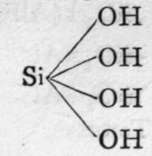
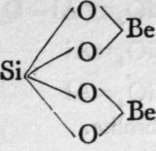
Beryl.
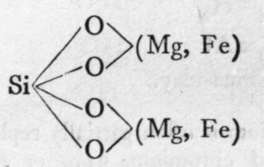
Olivine.
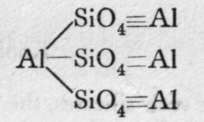
Xenolite.
Continue to:
