Silicic Acids And Silicates
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Silicic Acids And Silicates
While the characteristic of carbon is to form compounds in which many atoms of carbon are linked together (hydrocarbons, for example, having formulas like '. , atoms of silicon are characterised by linking by means of atoms of oxygen. This peculiarity leads to the existence of a large number of silicates, and probably, too, of a large number of silicic acids. The existence of some of these is rendered certain by a study of the oxychlorides.
, atoms of silicon are characterised by linking by means of atoms of oxygen. This peculiarity leads to the existence of a large number of silicates, and probably, too, of a large number of silicic acids. The existence of some of these is rendered certain by a study of the oxychlorides.
Silicon tetrachloride, SiCl4, when passed over fragments of felspar (a silicate of aluminium and calcium) heated to whiteness in a porcelain tube, exchanges chlorine for oxygen, and yields a liquid boiling at about 137°, of the formula
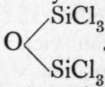 This liquid, passed along with oxygen through a hot glass tube, gave two other liquids, which could be separated by fractionation; the one, boiling at 153°, had the formula Si4O3Cl10, and the other, boiling at 200°, Si4O4Clg. The vapour-densities of these liquids were determined, and led to the formulae given above. The signification of this will appear presently.
This liquid, passed along with oxygen through a hot glass tube, gave two other liquids, which could be separated by fractionation; the one, boiling at 153°, had the formula Si4O3Cl10, and the other, boiling at 200°, Si4O4Clg. The vapour-densities of these liquids were determined, and led to the formulae given above. The signification of this will appear presently.
Si(OH)4. When silica, in the form of flint, or fine sand, or powdered rock-crystal is either fused with caustic soda or potash, or heated under pressure with a solution of one or other of the alkalies, an orthosilicate is produced, possessing the formula Si(ONa)4 or Si(OK)4. These silicates are soluble in water, and as they resemble glass in appearance, they are usually named " soluble glass." If hydrochloric acid is added to the solution of one of them, no apparent change occurs; in reality, orthosilicic acid is produced, a compound which is hardly ionised at all, being one of the very weakest of acids.
Continue to:
- prev: Acids Containing Carbon
- Table of Contents
- next: Osmosis
