Ferro- And Ferricyanides
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Ferro- And Ferricyanides
Some of the double cyanides are of importance, both commercially and from a chemical standpoint. Among them is a" substance which has already been mentioned, potassium ferrocyanide,
K4Fe(CN)6. This compound forms large yellow tabular crystals; it contains ferrous iron, hence the name ferro(us)cyanide. It is supposed to be derived from a tricyanogen group, 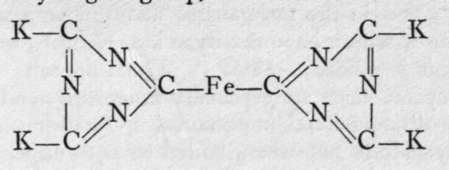 and to have the formula
and to have the formula
The ions of this salt are live, viz., 4K, and the complex group =Fe(CN)c ; the fall in freezing-point of an aqueous solution caused by the presence of a gram-molecule in 100,000 parts of water, and the conductivity of a similarly dilute aqueous solution indubitably indicate the presence of five ions. Moreover, the salt shows none of the reactions characteristic of ions of dyad iron, such as precipitation as sulphide on addition of ammonium sulphide, precipitation as hydroxide on addition of alkalies, etc. The acid corresponding to this salt, hydroferrocyanic acid, H4Fe(CN)6, can be prepared by adding to a boiled solution of potassium ferrocyanide concentrated hydrochloric acid and a little ether; it precipitates in white crystals. The zinc salt and the copper-potassium salt, K2CuFe(CN)6, are insoluble ; the former is white, and the latter a slimy brown-red precipitate.
On passing a current of chlorine through a solution of potassium ferrocyanide, or on submitting it to the action of any oxidising agent, potassium ferri(c) cyanide is formed: 2K4Fe(CN)fl. Aq + Cl2 = KC1. Aq + 2K8Fe(CN)fl. Aq. The new compound contains ferric iron, hence its name; the " c99 is omitted for the sake of euphony. This salt crystallises in dark red prisms, and dissolves in water with an orange colour. The acid, prepared from the lead salt, which is sparingly soluble, by the action of dilute sulphuric acid, and evaporation to crystallising point, forms brownish needles. Here again the complex group =Fe(CN)G is one of the complex ions in solution along with 3K ; and it is to be noticed that it now carries only three electrons instead of four, as in the ferrocyanide. Similar instances are to be remarked in elements of two valencies; and in the manganates and the permanganates, the former of which have the dyad ion =MnO4, while with the latter it is monad, -MnO4. The iron salts of ferro-and ferricyanic acids are especially interesting, and some of them are of commercial importance. On adding a solution of ferrocyanide of potassium, boiled so as to expel dissolved oxygen, to a solution of iron wire in sulphurous acid, which is also free from dissolved oxygen, a white precipitate of potassium ferrous ferrocyanide results: FeSO3. Aq + K4Fe"(CN)6.Aq = K2Fe"Fe"(CN)6 + K2SO8.Aq. If these precautions to exclude oxygen are not taken, the precipitate is light blue in colour, and is a common test for ferrous iron. This compound is also formed when ferrocyanide of potassium is distilled with dilute sulphuric acid, as in the preparation of prussic acid: 2K4Fe"(CN)6.Aq + 3H2SO4.Aq m K2Fe"Fe"(CN)0 + 3K2SO4. Aq + 6HCN. When boiled with dilute nitric acid, the white compound is converted into a blue soluble compound, which may be regarded either as potassium ferrous ferricyanide or potassium ferric ferrocyanide, KFe"Fe'"(CN)6, or KFe"'Fe"(CN)G. This same compound is produced also by the addition of a ferric salt to potassium ferrocyanide: K4Fe"(CN)G.Aq + Fe,,,Cl3.Aq = KFe"'Fe"(CN)6.Aq + 3KCl.Aq ; or by adding a ferrous salt to potassium ferricyanide : K3Fe"'(CN)6.Aq + Fe"Cl2.Aq = 2KCl.Aq + KFe"Fe'"(CN)6.Aq. When mixed with excess of a ferrous salt, it gives a blue precipitate named "Turnbull'sblue": 2KFe"Fe'"(CN)6.Aq + Fe"SO4.Aq = Fe"3Fe"'9(CN)1Q; and with excess of a ferric salt, "Prussian~blue"~is formed: 3KFe"Fe"'(CN)6.Aq +
![]() Potassium ferricyanide, with a ferric ion, gives a brown solution, which may contain ferric cyanide. These colours are used as tests for ferric or ferrous iron.
Potassium ferricyanide, with a ferric ion, gives a brown solution, which may contain ferric cyanide. These colours are used as tests for ferric or ferrous iron.
Chromicyanides, manganicyanides, cobalticyanides, ruthenocyanides, and osmocyanides, are also known, similar in formulae to the ferro- and ferri-cyanides. On the other hand, nickel and platinum form double cyanides similar in formula to K2Pt(CN)4. The platinum salts are very beautiful, possessing the property of dichroism, i.e. of transmitting light different in colour from that which the crystals reflect; moreover, only some of the facets of the crystals have this property.
rSilver cyanide is soluble in a solution of potassium cyanide, also forming a double salt, of the formula KAg(CN)2. Here the ions are K and -Ag(CN)2. This salt finds two uses. First, it is the compound from which silver is best deposited electrolytically in electroplating (see p. 10). Potassium auricyanide, KAu(CN)4.Aq, produced by the addition of auric chloride, AuClg, to a solution of potassium cyanide, is employed in gold-plating. Second, the existence of the soluble ion, Ag(CN)9, furnishes a means of estimating the amount of hydrocyanic acid in a dilute solution such as is used for medicinal purposes. A decinormal solution of silver nitrate, that is, one containing one-tenth of the molecular weight of the salt taken in grams, or 17 grams per litre, will react with 13.02 grams of potassium cyanide, or with 5.4 grams of hydrocyanic acid, forming the double salt, thus :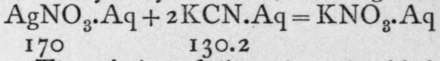
![]() The solution of silver nitrate is added trom a measuring-tube or burette until a faint trace of turbidity begins to appear; this signifies that the reaction given above has completed itself, and that the second reaction - KAg(CN)2. Aq + AgNO3. Aq = KNO8. Aq +
The solution of silver nitrate is added trom a measuring-tube or burette until a faint trace of turbidity begins to appear; this signifies that the reaction given above has completed itself, and that the second reaction - KAg(CN)2. Aq + AgNO3. Aq = KNO8. Aq +
2AgCN-has just begun. Every cubic centimetre, therefore, of silver nitrate added corresponds to the presence of 0.0054 gram of hydrocyanic acid in solution.
Metallic gold is soluble in a dilute solution of potassium or sodium cyanide, the complex group Au(CN)3 being formed, thus:- 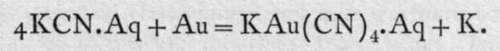
The action of the potassium on the water is to liberate hydrogen. But this hydrogen attacks the oxygen dissolved in the water, and is removed as water. The process is largely used in recovering gold from poor gold ores, or from the " slimes," or mud left after removing the bulk of the gold from the crushed ore, by amalgamating it with mercury.
The addition of a solution of potassium cyanide to a solution of a cupric salt in ammonia, which, it will be remembered, contains the blue ions of the cupramine, = Cu(NH3)2, decolorises the solution. This is, due to the formation of the double salt, potassium cupricyanide, K2|=Cu(CN)4.Aq, the ions of which are colourless. The copper is not present in the form of cupric ions, Cu", hence it does not give the reactions characteristic of these ions. For example, it yields no precipitate with sulphuretted hydrogen; and this affords a means of separating copper from cadmium, which is unaffected by addition of potassium cyanide.
Continue to:
