Chapter X. Borides And Carbides
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Chapter X. Borides And Carbides
These compounds have been incidentally mentioned on p. 30; they have been investigated almost exclusively by Moissan and his pupils.
Borides
Calcium, strontium, and barium borides have been prepared by heating in an electric furnace a mixture of borate of the metal with aluminium filings and carbon. At the high temperature of the electric arc the carbon reduces the aluminium oxide and prevents its formation. These compounds form hard, transparent microscopic cubes, burning only when maintained at a red heat in oxygen, and attacked with difficulty by the halogens. Their formulae are curious; they are analogous to the very unstable hydrazoates, M'(N)3, being Ca(B3)2, Sr(B3)2, and Ba(Bg)2; and their existence would point to a supposititious compound of the formula 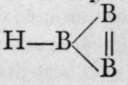 like
like
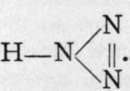
Ferric boride, produced by heating together boron and wrought-iron in an electric furnace, consists of brilliant yellowish-grey crystals, burning brilliantly when heated in oxygen, and attacked by nitric acid. The corresponding compounds of nickel and cobalt, prepared in the same manner, form brilliant prisms. The formulas are FeB, NiB, and CoB.
Carbon boride, CB6, forms lustrous black crystals, nearly as hard as diamond, on which facets can be cut by its use; it is produced by heating a mixture of amorphous boron and sugar-charcoal in the electric furnace.
Continue to:
