Isomeric Cyanides
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Isomeric Cyanides
The formula of hydrocyanic acid can be represented in two ways. It is possible to conceive either the carbon or the nitrogen to be united with the atom of hydrogen. In the former case, the structural formula is H-C=N; in the latter, H-N=C. There is no method of determining which of the two formulae is to be ascribed to the acid or to its simple salts; but salts with alcohol radicals are known to which one or other formula can be ascribed. On distilling potassium cyanide with potassium ethyl-sulphate, the following change takes place: K-C=N + C2H5.KSO4 = CH3-CH2-C-N + K2SO4. Here it is known that the carbon of the ethyl group is in direct union with the carbon of the cyanogen for two reasons: first, when ethyl cyanide is exposed to the action of nascent hydrogen (e.g. treated with tin and hydrochloric acid) hydrogen adds itself both to the carbon and to the nitrogen of the cyanide group, and propylamine, CH3-CH2-CH2-NH0, is formed ; and second, on boiling ethyl cyanide with a solution of caustic potash in alcohol, an acid with three carbon atoms, propionic acid, is formed : ![]()
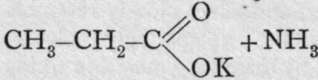 ; dyad oxygen and monad potassoxyl replace triad nitrogen. On the other hand, if ethyl iodide, CH3-CH2-I, be boiled in alcoholic solution with silver cyanide, the changeis:
; dyad oxygen and monad potassoxyl replace triad nitrogen. On the other hand, if ethyl iodide, CH3-CH2-I, be boiled in alcoholic solution with silver cyanide, the changeis: ![]()
![]() Here the nitrogen is in direct union with the carbon atom of the ethyl group ; this is known because on boiling the compound with dilute acid, hydrolysis takes place, thus:
Here the nitrogen is in direct union with the carbon atom of the ethyl group ; this is known because on boiling the compound with dilute acid, hydrolysis takes place, thus: ![]()
![]() the nitrogen remains in union with the carbon. Hence it is concluded that while potassium cyanide must contain K-C-N, along with
the nitrogen remains in union with the carbon. Hence it is concluded that while potassium cyanide must contain K-C-N, along with ![]() (for both compounds are formed by the first action), silver cyanide consists almost exclusively of Ag-N=C. The name applied to the first compound, CH3CH2CN, is ethyl cyanide, or, preferably, propionitrile, seeing that it differs from propionic acid only in having nitrogen in place of oxygen and hydroxyl; while the latter is termed ethyl isocyanide or ethyl carbamine, for it contains carbon replacing hydrogen in ethylamine,
(for both compounds are formed by the first action), silver cyanide consists almost exclusively of Ag-N=C. The name applied to the first compound, CH3CH2CN, is ethyl cyanide, or, preferably, propionitrile, seeing that it differs from propionic acid only in having nitrogen in place of oxygen and hydroxyl; while the latter is termed ethyl isocyanide or ethyl carbamine, for it contains carbon replacing hydrogen in ethylamine, ![]()
Hydrocyanic acid may on the same grounds be termed " formonitrile," for, on standing with dilute acid, it is converted into formic acid by assumption of the elements of water: ![]() and cyanogen, iled "oxalonitrile " :
and cyanogen, iled "oxalonitrile " : ![]()
Continue to:
