Ethers
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Ethers
Oxide of methyl and oxide of ethyl, which are usually named methyl and ethyl ethers, are formed by mixing solutions in alcohol of methyl or ethyl iodide with sodium methoxide or ethoxide : CH3I. Ale + NaOCHg. Ale = NaI + H3COCH3.Alc. The ether has a low boiling-point, and can be separated by fractional distillation from the alcohol in which it is dissolved. Methyl ether is a gas; ethyl ether a volatile liquid, boiling at 37°. Such compounds can also be prepared more readily by distilling a mixture of the alcohol with sulphuric acid, which yields HCH3SO4, hydrogen methyl sulphate, with the alcohol: HCH.,SO4 + CH3OH = H3COCH3 + H2SO4. Now, methyl ether and hydrochloric acid combine at a low temperature, yielding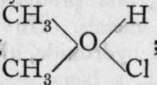 ; but it is impossible to replace the chlorine by hydroxyl.
; but it is impossible to replace the chlorine by hydroxyl.
Similar sulphur compounds, however, are stable. Methyl sulphide, produced bv the action of methvl iodide on potassium sulphide,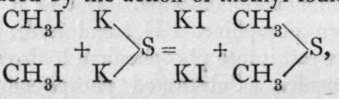 unites with methyl iodide, forming
unites with methyl iodide, forming![]() a compound containing tetrad sulphur; with silver hydroxide it yields the corresponding tri-methyl-sulphonium hydroxide,
a compound containing tetrad sulphur; with silver hydroxide it yields the corresponding tri-methyl-sulphonium hydroxide,
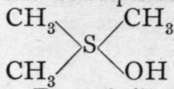 , a compound exhibiting basic properties.
, a compound exhibiting basic properties.
From iodine, too, iodonium compounds have been prepared, in which the iodine functions as a triad; and a hydroxide with basic properties is known.
Continue to:
