Sulphur Trioxide
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Sulphur Trioxide
The constitution of sulphuryl chloride and its conversion into sulphuric acid has already been alluded to. And it may be assumed that that of sulphur trioxide, SO3, is expressed by the formula 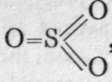 , sulphur acting as a hexad. Although sulphur dioxide unites directly with chlorine, it does not combine with oxygen, unless the two gases are brought intimately into contact by passing them over finely divided platinum ; such platinum is best prepared by dipping asbestos (a native magnesium silicate, possessing a fibrous structure) into platinic chloride, and subsequent ignition, when the chloride is decomposed into chlorine, which escapes, and a deposit of spongy platinum on the asbestos. On a large scale, sulphur dioxide, made by burning sulphur or iron pyrites, FeS2, in air, is concentrated by solution in water, the gas being forced in under some pressure ; the solution, on being exposed to reduced pressure, gives up the gas, which is thus freed from the nitrogen of the atmosphere. The sulphur dioxide is then mixed with air and passed over the platinised asbestos heated to a definite high temperature. Combination ensues, and the sulphur trioxide is condensed in cooled receivers. It is a white, crystalline, fuming substance, dissolving in water with a hissing noise and with great evolution of heat. It also unites directly with hydrogen chloride, with formation of chloro-sulnhonic acid, CI-SO2-OH, a fuming very corrosive liquid (see p. 151).
, sulphur acting as a hexad. Although sulphur dioxide unites directly with chlorine, it does not combine with oxygen, unless the two gases are brought intimately into contact by passing them over finely divided platinum ; such platinum is best prepared by dipping asbestos (a native magnesium silicate, possessing a fibrous structure) into platinic chloride, and subsequent ignition, when the chloride is decomposed into chlorine, which escapes, and a deposit of spongy platinum on the asbestos. On a large scale, sulphur dioxide, made by burning sulphur or iron pyrites, FeS2, in air, is concentrated by solution in water, the gas being forced in under some pressure ; the solution, on being exposed to reduced pressure, gives up the gas, which is thus freed from the nitrogen of the atmosphere. The sulphur dioxide is then mixed with air and passed over the platinised asbestos heated to a definite high temperature. Combination ensues, and the sulphur trioxide is condensed in cooled receivers. It is a white, crystalline, fuming substance, dissolving in water with a hissing noise and with great evolution of heat. It also unites directly with hydrogen chloride, with formation of chloro-sulnhonic acid, CI-SO2-OH, a fuming very corrosive liquid (see p. 151).
Continue to:
