Molybdates, Tungstates, And Uranates
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Molybdates, Tungstates, And Uranates
The formulae of the molybdates, tungstates, and uranates are analogous to those of the chromates; for example, K2MoO4, Na2WO4, (NH4)2UO4. The common ore of molybdenum is the disulphide, crystalline scales resembling graphite, MoS2, termed molybdenite. On heating it in the air, or on boiling it with concentrated nitric acid, it is oxidised to the trioxide, MoO3, a white slippery powder. Wolfram, (Fe,Mn)WO4, is the chief ore of tungsten; on boiling with concentrated nitro-hydrochloric acid, calcium nitrate and chloride go into solution, and tungstic acid, H2WO4, remains as an insoluble yellow powder. On heating it, it loses water, and yields the anhydride, a powder with similar colour, WOg. Pitchblende is the name of the commonest ore of uranium; its formula is U3O8. On fusing it with a mixture of nitrate and carbonate of soda, sodium uranate Na2UO4 is formed ; and on adding acid, uranic acid, H2UO4 is precipitated, as a yellow powder. On heating it to 3000, a scarlet powder, of the formula UO3, remains. Ignition changes it into U3Og, possibly uranium uranate, U(UO4)2, of the same formula as the natural mineral. The chief molybdate is that of ammonium, (NH4)2MoO4, white crystals obtained by dissolving the acid in ammonia solution; it is used in precipitating phosphoric acid as phospho-molybdate of ammonium, a representative of many very complicated molybdates ; its formula is 16MoO3.P2O5.3(NH4)2O.14H2O; it is a derivative of one of the condensed molybdic acids. Sodium tungstate, Na2WO4, produced by fusing the trioxide with sodium carbonate, is used as a mordant in dyeing, and it has the property of rendering cotton and linen fabrics uninflammable. The chief characteristic of uranium trioxide is that of forming uranyl salts, such as uranyl nitrate, (UO2)(NO3)2, and acetate, (UO2) (C2H3O2)2, where uranyl,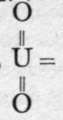 acts as a dyad radical. The uranates are ill-defined compounds.
acts as a dyad radical. The uranates are ill-defined compounds.
Continue to:
