Highly Oxidised Acids
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Highly Oxidised Acids
Of recent years, a considerable number of salts of acids more highly oxidised than any of those already mentioned has been prepared. It has long been known that on addition of hydrogen dioxide to a solution of potassium bichromate acidified with sulphuric acid, a bright blue colour is produced, and that this coloured substance can be extracted by ether from its aqueous solution. The compound has recently been identified as perchromic acid, CrO4(OH); for, on adding to the cooled blue solution a solution of ammonia in ether, a violet precipitate of CrO4(0-NH4).H2O2 is thrown down; and if an ethereal solution of potassium hydroxide be added, the potassium salt of similar formula is precipitated. These bodies are explosive.
Persulphates of potassium and ammonium are produced by passing a current of electricity through concentrated solutions of the sulphates in water. The persulphate is sparingly soluble, and deposits in white crystals. The formula appears to be M2S2Og (M = monad metal). The acid has bleaching powers, and gradually decomposes into sulphuric acid and ozone.
Perborate of sodium, NaBO3,4H2O, is similarly prepared, or it may be produced by cooling a solution of borax to which some caustic soda and hydrogen peroxide have been added. It, too, is a sparingly soluble salt, possessing bleaching properties.
Percarbonate of sodium, Na2CO4. i^H2O, is similarly prepared by addition of alcohol to a solution of sodium carbonate, to which a solution of hydrogen peroxide has been added. It is a white, extremely unstable compound, possessing, like the other similar salts, great oxidising power.
All these silts are probably constituted like hydrogen dioxide, H-O-O-H : for example, the percarbonate may be regarded as 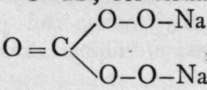 , and not i
, and not i ![]() , where the carbon would be octavalent; carbon appears never to function with a higher valency than that of a tetrad. Expressed in terms of electrons, carbon can receive or part with only four.
, where the carbon would be octavalent; carbon appears never to function with a higher valency than that of a tetrad. Expressed in terms of electrons, carbon can receive or part with only four.
Continue to:
- prev: Hydrosulphites
- Table of Contents
- next: Chapter IX. The Nitrides And Phosphides, Arsenides And Antimonides
