Chapter IX. The Nitrides And Phosphides, Arsenides And Antimonides
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Chapter IX. The Nitrides And Phosphides, Arsenides And Antimonides
Analogy Between Oxides And Nitrides
Nitrogen and phosphorus are best characterised by the compounds in which they act as triads. For just as an oxide or hydroxide may, as was customary during the era of the theory of " types," be regarded as water in which the atoms of hydrogen are more or less completely replaced by atoms of a metal; so from analogy it is to be inferred that compounds should be preparable which should be similarly related to ammonia and to hydrogen phosphide. The following graphic formulas will render the conception clear:-
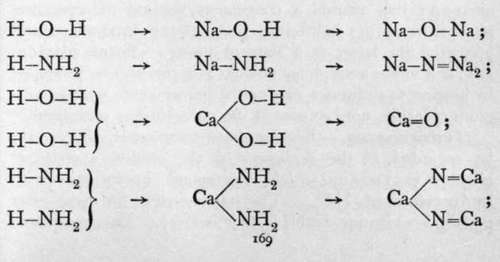
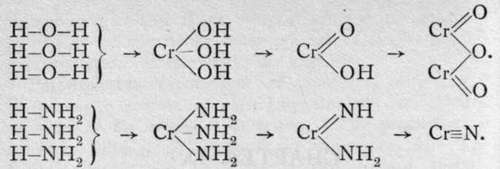
But although most elements combine with oxygen directly, there are only a few which burn in nitrogen. Among these are lithium, calcium, and magnesium; boron and titanium also possess this property. The nitrides of the other elements are practically unknown. These nitrides are attacked by water; the three first with violence at the ordinary temperature; the two last, when heated in a current of steam. The products are the hydroxide of the metal and ammonia; or with boron and titanium, owing to the high temperature of action, the oxide, thus: Mg3N2 + 3H2O.Aq = 3Mg(OH)2 + 2NH8.Aq.
Continue to:
- prev: Highly Oxidised Acids
- Table of Contents
- next: Nitrides
