Nitrides
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Nitrides
Lithium nitride, Li3N, is a dark-coloured substance; it is formed at the ordinary temperature on exposing metallic lithium to the air. Calcium nitride, Ca3N2, is a greyish-yellow substance; and magnesium nitride, Mg3N2, a yellow powder. Combination takes place readily with great evolution of heat when a mixture of dry lime with magnesium powder is heated to dull redness in a current of nitrogen; this affords a convenient method of separating nitrogen from the indifferent gases of the atmosphere, and preparing the latter in a state of purity. Boron nitride, BN, is a white amorphous powder ; it can also be produced by heating to redness a mixture of boron oxide with ammonium chloride, until excess of the chloride has volatilised.
Hydrazoates
Besides these compounds, which may be regarded as the analogues of the oxides, a series of nitrides is known, which correspond in formula with hydrazoic acid, HN3. The starting-point for these compounds is sodamine, NaNH2 (see below). This compound is heated to 300° in a series of small flasks in a current of nitrous oxide, when the following reaction takes place: 2NaNH2 + N2O = NaN3 + NaOH + NH3. The change which takes place is more obvious when the reaction is conceived to occur in two stages: 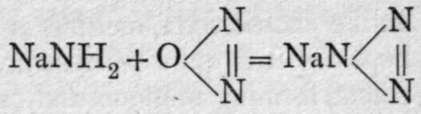
+ H2O; and NaNH2 + H2O = NaOH + NH?. The product of the reaction is dissolved in water, acidified with dilute sulphuric acid, and distilled : NaNg.Aq + H2SO4. Aq = HN3.Aq + NaHSO4.Aq. The distillate, which is a dilute solution of hydrazoic acid, has a peculiar odour, and if its vapour be inhaled, fainting may result; it is necessary to take precautions to distil it in a good draught. The solution has an acid reaction; salts may be prepared by neutralisation with the hydroxides or carbonates of the metals. The ions, - N3, are colourless, and the salts of colourless ions are themselves white. Those of lithium, sodium, potassium, magnesium, calcium, strontium, barium, and zinc are crystalline; their formulas are M'N3 and M/;(N3)2 respectively. Silver hydrazoate, AgN3, closely resembles the chloride in appearance and in insolubility ; it is, however, dangerously easy to explode, and should be prepared dry only in minute quantity, and treated with the utmost precaution. Titration with a deci-normal solution of silver nitrate affords a convenient method of determining the strength of a solution of hydrazoic acid, or of analysing the hydrazoates; it is easy to recognise the point when all hydrazoic acid has been removed as the insoluble silver salt.
Continue to:
- prev: Chapter IX. The Nitrides And Phosphides, Arsenides And Antimonides
- Table of Contents
- next: Amines
