Acid Chlorides
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Acid Chlorides
Sulphur dioxide combines with chlorine when a mixture of the two gases is exposed to sunlight, or when it is passed over gently heated charcoal.
The product, sulphuryl chloride, 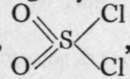 , is a colourless fuming liquid, boiling at 7 70. On adding it to water, it immediately yields sulphuric acid bv replacement of the chlorine by hydroxyl:
, is a colourless fuming liquid, boiling at 7 70. On adding it to water, it immediately yields sulphuric acid bv replacement of the chlorine by hydroxyl: ![]()
Selenium and tellurium form similar compounds; and so also do molybdenum, tungsten, and uranium, as well as chromium. Molybdyl, tungstyl, and uranyl chlorides are produced by passing chlorine over the dioxides heated to redness ; they are not decomposed by water, but when boiled with alkalies they are converted into molybdates, tungstates, or uranates. Chromyl chloride, on the other hand, is formed by distilling together a chromate, a chloride, and concentrated sulphuric acid. This amounts to the action of hydrogen chloride on chromium trioxide, thus: CrO3 + 2HCl = CrO2Cl2-f-H2O. The presence of the sulphuric acid is necessary in order to withdraw and retain water, for chromyl chloride is at once attacked by water, chromic acid being formed. It is a deep red fuming liquid, hardly distinguishable from bromine in appearance ; it boils at 118°. A manganyl chloride is said also to have been prepared.
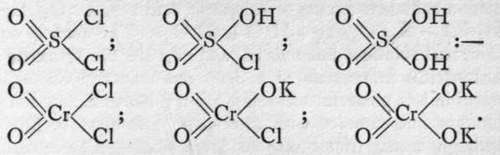
The constitution of the acids is inferred from that of the chlorides ; and in the case of chromium, an intermediate body is known between chromyl chloride and potassium chromate, termed chlorochromate ; its formula is
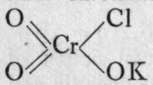 ; with sulphur, the corresponding acid, chlorosulphuric, or, better, chlorosulphonic acid is known,
; with sulphur, the corresponding acid, chlorosulphuric, or, better, chlorosulphonic acid is known,
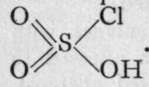 These bodies are produced by the method of mixture; the former by crystallising together anhydro-chromateand chloride of potassiun
These bodies are produced by the method of mixture; the former by crystallising together anhydro-chromateand chloride of potassiun ![]()
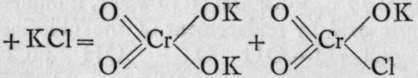 ; the latter, by the nninn nf nvHrnrhlnrir nri'H with sulphur trioxide, thus :
; the latter, by the nninn nf nvHrnrhlnrir nri'H with sulphur trioxide, thus :
The former consists of red crystals ; the 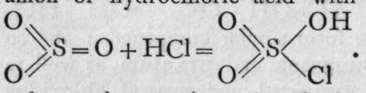 latter is a fuming liquid, readily acted on
latter is a fuming liquid, readily acted on
CHROMATES by water, with formation of sulphuric and hydrochloric acids. We have thus with sulphur and with chromium the series:
Continue to:
- prev: Isomeric Sulphites
- Table of Contents
- next: Chromates
