Chromic Acid
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Chromic Acid
Chromic acid is liberated on adding to a concentrated solution of potassium anhydrochromate a sufficient excess of sulphuric acid : K2Cr2O7. Aq -f H2SO4 = K2SO4.Aq + H2O + 2CrO3. The acid, in concentrated solution, loses water, and deposits the trioxide or anhydride in crystals of a deep red colour. Chromium trioxide is a powerful oxidising agent; hence it may not be brought into contact with filter-paper ; it must be filtered through a mat of asbestos or glass wool. The excess of sulphuric acid and potassium sulphate are washed out with concentrated nitric acid, in which the anhydride is almost insoluble ; the nitric acid is then volatilised by gentle heat. This anhydride dissolves in water, but it is doubtful whether the acid H0CrO4 is contained in the solution ; it is more probable that the ions are HH and =Cr2O7, from the colour, and other tests, such as the conductivity.
Oxidation by means of a solution of chromic anhydride is carried out either by boiling the substance to be oxidised with a mixture of bichrome and dilute sulphuric acid, or with a solution of chromic anhydride in pure acetic acid; the chromate ion, =CrO4 or =Cr2O7, is changed into the chromic ion Cr ; the action is :-
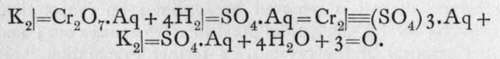
If the sulphuric acid is hot and concentrated, oxygen is evolved as gas; if dilute, substances present in solution, if they are capable of being oxidised, are attacked by the oxygen. When chromic anhydride is heated, it is converted into chromium sesquioxide, Cr2O3, with evolution of oxygen.
Continue to:
- prev: Chromates
- Table of Contents
- next: Manganates
