Section IV. Chemical Changes On Sensitive Preparations Of Bromide Of Silver
Description
This section is from the book "A Manual Of Photography", by Robert Hunt. Also available from Amazon: A Manual of Photography.
Section IV. Chemical Changes On Sensitive Preparations Of Bromide Of Silver
In many of the works on chemistry, it is stated that the chloride is the most sensitive to light of all the salts of silver ; and, when they are exposed in a perfectly formed and pure state to solar influence, it will be found that this is nearly correct. Modern discovery has, however, shown that these salts may exist in peculiar conditions, in which the affinities are so delicately balanced as to be disturbed by the faintest gleam ; and it is singular that, as it regards the chloride, iodide, and bromide of silver, when in this condition, the order of sensibility is reversed, and the most decided action is evident on the bromide before the eye can detect any change in the chloride.
The slight additional expense of the bromides is not worthy-consideration, particularly as their use may be confined to papers for the camera obscura, the pictures on which are of course of the negative character, and the positive photographs can be formed by transfer on the chloridated papers of a highly sensitive kind. Since there has been some question as to the use of the iodide of silver without an infringement of patent, it is not a little surprising that the bromide has not been more generally employed.
It will be found that the bromide and iodide are much alike in the singular want of sensibility which they sometimes exhibit under the circumstances already alluded to, which are not easy of explanation.
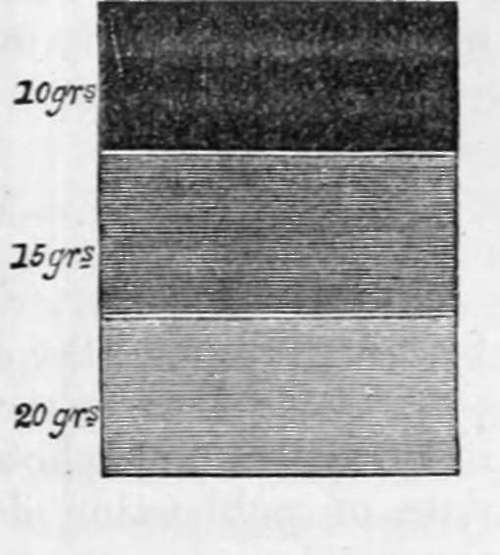
If a paper first washed with a solution of nitrate of silver has bromide of potassium applied to it in different proportions, say 20 grains, 15 grains, and 10 grains each, in two drachms of water, and, when dry, be again washed over with the silver solution, it will be found, unless, as is occasionally the case, some organic combination interferes, that the weakest solution, the strongest being the least influenced by light. The different degrees of darkness induced are fairly represented in the margin. (Fig. 18.) As the different bromides give to photographic paper varieties which much resemble those enumerated under the muriates, I have thought it unnecessary to give an account of any of them. The paper prepared with the bromide of potassium is the kind I have adopted, after having tried upwards of two hundred combinations of silver with the other bromides.
To prepare a highly sensitive paper of this kind, select some sheets of very superior glazed post, and wash it on one side only with bromide of potassium—forty grains to one ounce of distilled water, over which, when dry, pass a solution of one hundred grains of nitrate of silver in the same quantity of water. The paper must be dried as quickly as possible without exposing it to too much heat; then again washed with the silver solution, and, when dry, carefully preserved for use.
It will be perceived that I adopt a slightly different manipulation from that recommended by Mr. Talbot. Instead of washing the paper with the solution of silver first, and applying the bromide or the muriate over this, and then the silver wash again, I use the alkaline salt first, and apply the metallic washes one on the other. I have been induced to this from observing that the photographic preparation penetrates less deeply into the paper than when laid on as originally prescribed, and, consequently, the sensibility of it is increased. It will be found that an addition of about one-twelfth of spirits of wine to the solution of silver will much increase the blackness of the paper when solarised ; and I think we may safely say that the sensibility is also improved by it,—at all events it is not impaired.
M. Biot has expressed his opinion that it is not possible to find any substance more sensitive to light than the bromide of silver : this is true to a certain extent, but in combination with deoxidizing agents other preparations will be named which have a decided superiority over the pure bromide of silver.
Continue to:
- prev: Section III. Chemical Changes On Sensitive Preparations Of Iodide Of Silver
- Table of Contents
- next: Section V. Chemical Changes On Sensitive Preparations Of Miscellaneous Salts Of Silver
