Section I. Nitrate Of Silver
Description
This section is from the book "A Manual Of Photography", by Robert Hunt. Also available from Amazon: A Manual of Photography.
Section I. Nitrate Of Silver
The most simple kind of photographic paper which is prepared with the silver salts is that washed with the nitrate of silver only; and for many purposes it answers well, particularly for copying lace or feathers; and it has this advantage over every other kind, that it is perfectly fixed by well soaking in warm water, free from chloride of sodium.
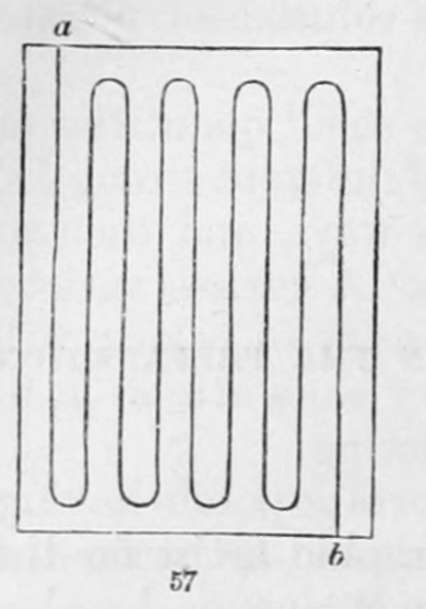
The best proportions in which this salt can be used are 60 grs. of it dissolved in a fluid ounce of distilled water. Care must be taken to apply it equally, with a quick but steady motion over every part of the paper. It will be found the best practice to pin the sheet by its four corners to one of the flat boards above-mentioned, and then, holding it with the left hand a little inclined, to sweep the brush, from the upper outside corner, over the whole of the sheet, removing it as seldom as possible. The lines in fig. 57 will represent the manner in which the brush should be moved over the paper, commencing at a and ending at 6. On no account must the lines be brushed across, nor must we attempt to cover a spot which has not been wetted, by the application of fresh solution to the place, as it will, in darkening, become a well defined space of a different shade from the rest of the sheet. The only plan is, when a space has escaped our attention in the first washing, to go over the whole sheet with a more dilute solution. It is, indeed, always the safest course to give the sheet two washings.
The nitrated paper not being very sensitive to luminous agency, it is desirable to increase its power. This may be done to some extent by simple methods.
By soaking the paper in a solution of isinglass or parchment size, or by rubbing it over with the white of egg, and drying it prior to the application of the sensitive wash, it will be found to blacken much more readily, and assume different tones of colour, which may be varied at the taste of the operator.
By dissolving the nitrate of silver in rectified spirits of wine, instead of water, we produce a tolerably sensitive nitrated paper, which darkens to a very beautiful chocolate brown; but this wash must not be used on any sheets prepared with isinglass, parchment, or albumen, as these substances are coagulated by alcohol, and wash up forming streaks.
The nitrate of silver is not sufficiently sensitive to change readily in diffused light; consequently it is unfit for use in the camera obscura, and it is only in strong sunshine that a copy of an engraving can be taken in any moderate time.
Ammonio Nitrate Of Silver
This is an exceedingly useful preparation for many purposes. It is prepared by adding ammonia to a solution of nitrate of silver: a deep olive precipitate of oxide of silver takes place; more ammonia should then be added, drop by drop, until this precipitate is redissolved, great care being taken that no more ammonia is added than is necessary to effect a perfect solution of the oxide of silver. This solution is more sensitive than the nitrate, and may be used with advantage for copying by superposition; but it is not fitted for the camera obscura.
Continue to:
- prev: Chapter III. On The Modes Of Manipulation Adopted In The Preparation Of Sensitive Papers
- Table of Contents
- next: Section II. Chloride Of Silver
