Valency
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Valency
The Roman numerals below the vertical columns refer to what is termed the " valency." An element capable of combining with or replacing one atom of hydrogen, or, in other words, of which the equivalent and atomic weight are identical (see p. 15), is termed a monad, or is said to be monovalent. Thus, 23 grams of sodium replaces 1 gram of hydrogen in water or in hydrogen chloride, to form hydroxide or chloride of sodium; and as 23 is known to be the atomic weight of sodium from determinations of its specific heat, the atomic weight of sodium is expressed by the same number as its equivalent. It is therefore a monad. The element oxygen is a dyad or divalent, because, in water, two grams of hydrogen are combined with 16 grams of oxygen; its equivalent is therefore 8. But oxygen is 16 times as heavy as hydrogen—that is, a molecule of oxygen is 16 times as heavy as a molecule of hydrogen; and as a molecule of each of these substances is believed to consist of two atoms, an atom of oxygen is 16 times as heavy as an atom of hydrogen. The atomic weight is therefore 16 ; but as the equivalent is 8, the atomic weight is twice the equivalent. Hence the name " dyad." Similarly, there are tri-valent elements, or triads ; tetrava/ent elements, or tetrads ; pentavalent elements, or pentads ; hexavalent elements, or hexads ; heptavalent elements, or heptads ; and possibly one octovalent element, or octad.
Valency Of Elements
As elements may have more than one equivalent (see p. 15), so they may have more than one valency. Certain elements, however, so far as is known, possess only one valency; examples of this are found in the lithium, the beryllium, and the boron columns. But the majority of elements exhibit more than one valency, according to circumstances. Thus, compounds of nitrogen are known possessing the formulae NO, NH3, NO2, and NH4Cl, in which one atom of nitrogen is combined with one atom of dyad oxygen, and is therefore also a dyad; with three atoms of monad hydrogen, and is accordingly a triad ; with two atoms of dyad oxygen, whence nitrogen is here a tetrad ; and with four atoms of monad hydrogen and one atom of monad chlorine—in all, with five monads —and in this case nitrogen must be accounted a pentad. The atomic weight of nitrogen is known from its density to be 14; and its equivalents in these compounds are respectively -5-, V> an<^ V* This peculiarity makes the classification of some of the elements a difficult task.
But there is an additional difficulty which meets us in attempting to ascribe the valency to an element. It is connected with what is known as the " structure" of compounds. As this subject will be frequently alluded to in succeeding chapters, enough will only be said here to give an idea of the problem which faces us in attempting a rational classification of the elements.
We are ignorant of the form of the atoms. It is true that various speculations have been made which may possibly lead to a true conception of their appearance and motions, but these are not sufficiently definite and supported by facts to require more than a passing allusion here. For all practical purposes, we are content, in default of a better conception, to regard atoms as spheres, hard and elastic, and compounds as formed by the juxtaposition of these spheres. That this conception is far from reality is more than probable, but it has to suffice. Certain deductions, however, may be drawn regarding the methods of combination of the atoms in the molecule. It is certain that molecules must occupy space of three dimensions ; but just as it is possible to represent solid objects on a plane surface by the help of perspective, so it is allowable to picture molecules as made up of atoms spread over a plane surface, until we find facts which demand space of three dimensions. We shall see later that in certain cases such solid models of molecules are necessary, but, as a rule, they can be dispensed with. And instead of attempting to picture the atoms as circles or projected spheres, the symbols alone will be employed. The fact of combination will be indicated by a dash uniting the atoms; thus, a monad will have one, and only one, dash, proceeding from it; a dyad, two; a triad, three, and so on.
Structural Formulas
The simplest case which we can consider is that of a compound consisting of two monovalent atoms, such as hydrogen chloride. Here we have the structural formula, H—Cl. A compound of a dyad with two monad atoms, such as water, or its analogue, hydrogen sulphide, must have the formula, H—O—H, or H—S—H. The compound of a triad with three monad atoms, as, for example, ammonia, would be written 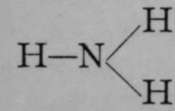 ; and of a tetrad with four monads,
; and of a tetrad with four monads, , where an atom of carbon is the tetrad, and the compound is named methane, or " marsh-gas." The atom of sulphur is, however, not always divalent; it is sometimes tetra-valent, as in its compounds with chlorine and with oxygen. In the first case, tetra-chloride of sulphur has the formula,
, where an atom of carbon is the tetrad, and the compound is named methane, or " marsh-gas." The atom of sulphur is, however, not always divalent; it is sometimes tetra-valent, as in its compounds with chlorine and with oxygen. In the first case, tetra-chloride of sulphur has the formula, 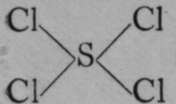 ; and in the latter, sulphur dioxide is represented by the formula
; and in the latter, sulphur dioxide is represented by the formula ![]() . Sulphur dioxide unites directly with chlorine on exposure of a mixture of the two gases to sunlight, forming a compound named sulnhuryl chloride, which has the empirical formula,
. Sulphur dioxide unites directly with chlorine on exposure of a mixture of the two gases to sunlight, forming a compound named sulnhuryl chloride, which has the empirical formula, ![]() ; in this compound sulphur is regarded as a hexad, hence the structural formula must be
; in this compound sulphur is regarded as a hexad, hence the structural formula must be 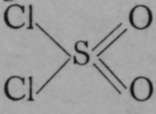 . Now, sulphuryl chloride reacts at once with water when they are brought into contact, and sulphuric acid is produced along with hydrogen chloride. This change can be represented structurally by the equation : —
. Now, sulphuryl chloride reacts at once with water when they are brought into contact, and sulphuric acid is produced along with hydrogen chloride. This change can be represented structurally by the equation : — 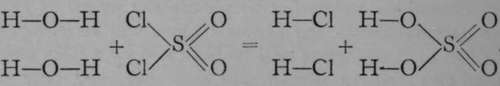 .
.
The chlorine atoms of the sulphuryl chloride have combined with two of the hydrogen atoms of two molecules of water, leaving the residues —O—H, which are termed " hydroxyl groups ; " these have taken the place of the chlorine atoms, forming sulphuryl hydroxide, or, as it is commonly termed, sulphuric acid. If the foregoing representation is correct, then an intermediate substance should exist, which may be named " sulphuryl hydroxy-chloride," and which should contain a chlorine atom and a hydroxyl group, each in union with sulphuryl. Such a body has been prepared by the direct union of sulphur trioxide, where sulphur is in combination with three atoms of oxygen, with hydrogen chloride. But here there must be a transposition of the hydrogen atom, as is evident from the equation—
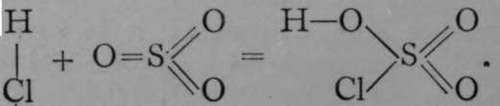
In a similar manner to the above schemes, the relations of the atoms in compounds may be traced out, but sometimes it is difficult to decide regarding the structure. Here is an instance. The specific heat of the element barium shows that it possesses an atomic weight not far removed from 137 ; the analysis of its chloride leads to the fact that 137/2 grams of barium are in combination with 35.5 grams of chlorine, and 35.5 is known to be the equivalent of chlorine; hence 63.5 is the equivalent of barium, and 63.5x2 = 137 is its atomic weight. Ordinary oxide of barium corresponds with this, for it contains 137 grams of barium in combination with 16 grams of oxygen; hence we accept barium as a dyad. But if barium oxide be heated to dull redness in a current of oxygen, another atom of oxygen combines with the oxide, and in the compound BaO0, 137 grams of barium are combined with 32 grams of oxygen. Is barium a tetrad ?
Among all the numerous compounds of barium, no one is known in which one atom of barium is combined with more than two atoms of a monad ; when barium dioxide is treated with hydrochloric acid, for example, two atoms of oxygen are not replaced by four atoms of chlorine, but the change is 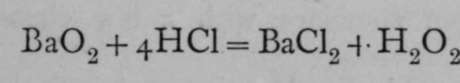 .
.
Hydrogen dioxide is produced. Now the formula of hydrogen dioxide has been proved by the freezing-point method to be H2O2, and not HO ; hence it may be supposed that it consists of two hydroxyl groups in union with each other, thus : H—O—O—H ; in this case, barium dioxide would be 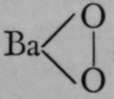 , the two atoms of oxygen being themselves united together ; and there are many instances of similar union. But it may also be held that one of the atoms of oxygen is a tetrad, the other remaining a dyad, thus :
, the two atoms of oxygen being themselves united together ; and there are many instances of similar union. But it may also be held that one of the atoms of oxygen is a tetrad, the other remaining a dyad, thus :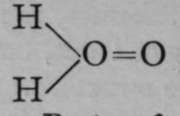 ; whence barium dioxide would be Ba=0=0. Both of these views can be supported by arguments, and it is an open question which has a claim to preference. It is certain, however, that barium is not a tetrad.
; whence barium dioxide would be Ba=0=0. Both of these views can be supported by arguments, and it is an open question which has a claim to preference. It is certain, however, that barium is not a tetrad.
In other instances, it must be confessed that the evidence is by no means so clear, and there is then considerable doubt as regards the correct classification of the elements concerned.
It must not be forgotten that we have as yet no clear conception as to the cause of valency ; at present we accept the facts, and endeavour to use them as a guide to the classification of compounds.
Were all the elements to be capable of combining with each other, it is easily seen that the number of compounds would be prodigious, and that no mind could possibly hope to grapple with them ; but it happens that only a certain number of elements forms well-defined compounds with the rest, and the grouping of compounds is thus not so difficult a task as might be supposed. The classes are the following :—
Continue to:
