Classification Of Compounds
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Classification Of Compounds
1. Hydrogen combines with a few elements, forming hydrides.
2. Fluorine, chlorine, bromine, and iodine combine with most elements, forming jluorides, chlorides, bromides, and iodides ; this group of elements is called the halogen group, and their compounds are often termed bolides.
3. Oxygen and sulphur also combine with most elements, and their compounds are named oxides and sulphides. The comparatively rare elements selenium and tellurium form similar compounds, named selenides and tellurides.
A very numerous and important class of compounds consists of those in which oxygen is combined partly with hydrogen, partly with another element. These compounds can be divided into two distinct classes, according to their behaviour in aqueous solution. Members of both classes are ionised, but they yield different ions according to the class to which they belong. An example of the first class is the compound H—O—CI, known only in solution in water, for it decomposes when an attempt is made to free it from water. The aqueous solution is onlv slightly ionised, but the ions present are ![]() and
and ![]() The hydrogen may be displaced by metals, forming " salts," which are also ionised, and indeed much more completely than H—O—CI. Thus we have K—O—CI, Ca=(0—Cl)2, and other similar salts, which are ionised in solution to
The hydrogen may be displaced by metals, forming " salts," which are also ionised, and indeed much more completely than H—O—CI. Thus we have K—O—CI, Ca=(0—Cl)2, and other similar salts, which are ionised in solution to ![]() and
and ![]() and toand
and toand ![]() respectively. Such hydroxides are named acids.
respectively. Such hydroxides are named acids.![]() .
.
It appears, however, that elements which form this class of hydroxide are, as a rule, incapable of retaining in combination many hydroxyl groups at a time ; hence compounds of this nature are generally mixed oxides and hydroxides. It might, for example, be imagined that triad nitrogen should be capable of retaining in combination three hydroxyl groups, to form 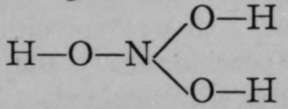 ; but the compound is unstable, and loses water, giving a mixed hydroxide and oxide, H—O—N=0. The ions in this case are
; but the compound is unstable, and loses water, giving a mixed hydroxide and oxide, H—O—N=0. The ions in this case are ![]() and
and ![]() . Another similar instance is that of sulphuric acid ; as it contains hexad sulphur, it might be supposed that the corresponding hydroxide of sulphur would be S(OH)6; but by loss of two molecules of water, sulphuric acid has the formula
. Another similar instance is that of sulphuric acid ; as it contains hexad sulphur, it might be supposed that the corresponding hydroxide of sulphur would be S(OH)6; but by loss of two molecules of water, sulphuric acid has the formula 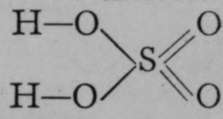 , as already shown. Its ions are
, as already shown. Its ions are ![]() , or sometimes
, or sometimes ![]() md
md ![]() The salts of these acids are respeclively m—o—n=0 and (m—0)2=SO4, where M' stands for any monad metal.
The salts of these acids are respeclively m—o—n=0 and (m—0)2=SO4, where M' stands for any monad metal.
Continue to:
