Thiosulphates
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Thiosulphates
Some other acids of sulphur remain to be noticed. Among these is thiosulphuric acid, H2S2O3, of which the sodium salt is produced by digesting together sodium sulphite with sulphur, just as, with oxygen, sodium sulphate is formed. In the latter case it may be supposed that the atom of oxygen inserts itself between the sodium atom and the sulphur atom with which it is in combination, thus :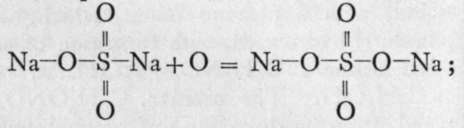
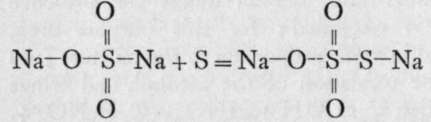 ; hence the name
; hence the name
" thio" sulphate, the " thion," or sulphur, replacing the oxygen of sulphuric acid. The sodium salt forms large transparent crystals of the formula Na2S2Os.5H2O ; the barium salt is sparingly soluble, and forms a crystalline precipitate on adding a solution of the sodium salt to one of barium chloride ; the lead salt is insoluble, and the silver salt is a white precipitate, which rapidly turns dark on application of heat, being converted into silver sulphide : Ag2S2O3 + H2O.Aq = Ag2S + H2SO4.Aq. On acidify-ing any one of the soluble salts, the acid is momentarily liberated ; but it immediately decomposes into sulphurous acid and sulphur, H2S2O3.Aq = H2SO3.Aq + S, the latter rendering the liquid milky. The sodium salt, when a solution of iodine in one of potassium iodide is added to it, undergoes the reaction : 2Na2S2O3. Aq + I2.Aq = 2NaI.Aq + Na2S4O6.Aq. The salt formed is named tetrathionate of sodium. It will be considered shortly.
Continue to:
