Iodometry
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Iodometry
A solution of sodium thiosulphate containing 248 grams, made up to a litre with water, reacts quantitatively with one containing 127 grams of iodine per litre ; the colour of the iodine disappears, and the vanishing of the last trace of iodine can be ascertained by the addition of some starch paste, which gives a blue colour so long as any free iodine remains unconverted into ions ; such a solution is commonly used in estimating iodine, or in determining the quantity present in solution of any substance which has the property of liberating iodine from acidified iodide, i.e. from hydriodic acid, such as free chlorine, a hypochlorite, or, indeed, any oxidising agent.
On boiling together solutions of sodium thiosulphate with ethyl iodide, sodium ethyl thiosulphate is formed ; its formula is 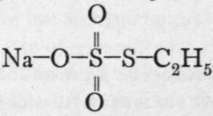 , for, when mixed with barium chloride, the barium salt, which is unstable, decomposes into barium dithionate
, for, when mixed with barium chloride, the barium salt, which is unstable, decomposes into barium dithionate ![]() and ethyl disulphide, thus :
and ethyl disulphide, thus :
This decomposition renders two suppositions probable: that thio-sulphuric acid has the formula 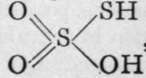 , and not
, and not ![]() and that dithionic acid is constitutionally represented by
and that dithionic acid is constitutionally represented by ![]()
Continue to:
- prev: Thiosulphates
- Table of Contents
- next: Hydrosulphites
