Isomerism. Polymerism. Part 3
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Isomerism. Polymerism. Part 3
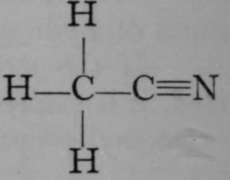
Acetonitrile.
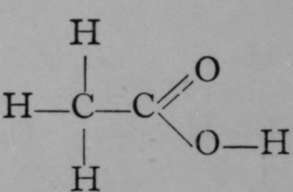
Acetic Acid.
It is seen to be closely allied to acetic acid, in which three of the atoms of hydrogen of ethane are also replaced ; but this time two by an atom of oxygen, and the third by the hydroxyl-group, —OH. This close connection is made obvious by boiling the acetonitrile with dilute alkali; the nitrogen is evolved as ammonia, while oxygen and hydroxyl take the place of the atom of nitrogen :—
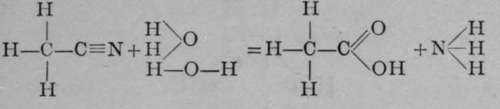
But an isomer of acetonitrile is also known, to which the formula 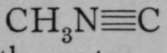 is ascribed; for, on causing it to react witn water, the products are methylamine, CH3—NH2, and formic acid,
is ascribed; for, on causing it to react witn water, the products are methylamine, CH3—NH2, and formic acid,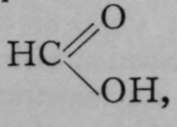 thus :—
thus :— 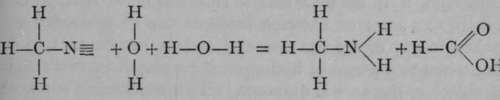 .
.
From this it is inferred that in the latter case the atom of nitrogen is in direct union with the atom of carbon of the CH3 group ; it remains united even after attack by water in presence of acid, the latter accelerating the action.
From these instances it is evident that the position of the elements or groups in a molecule, as well as the composition of the molecule, determine its nature ; and this fact is even more strikingly shown by isomerism in the group of compounds related to benzene, a liquid hydrocarbon of the formula C,.FL.
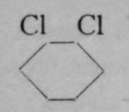
It is found that when this compound is attacked by chlorine, so that an atom of hydrogen is replaced by an atom of chlorine, the resulting oily liquid, named chlorobenzene, having the formula C(JH5Cl, exists in only one modification. But if two atoms of hydrogen are replaced by two atoms of chlorine, there are three compounds produced, to each of which the empirical formula C6H4Cl2 may be ascribed. To what is this isomerism due ?
The generally accepted graphic or structural formula for benzene, first suggested by Kekul£, lately professor of chemistry at Bonn, is in which the six atoms of carbon are arranged as a ring, and each in combination with one atom of hydrogen. Bearing in mind that a symmetrical replacement cannot produce isomerism, it is obvious that it is a matter of indifference whether an atom of chlorine replaces one of hydrogen attached to any of the six atoms of carbon, numbered i to 6. But when two atoms of hydrogen are replaced by two atoms of chlorine, the case is different. The substituting atoms of chlorine may have three distinct positions relatively to each other ; they may replace hydrogen atoms combined with the carbon atoms i and 2, or with i and 3, or, lastly, with 1 and 4. Obviously the numbering 1 and 2 expresses only any two contiguous atoms ; it is identical with 2 and 3, 3 and 4, etc. So, too, 1 and 3 is identical with 2 and 4, 3 and 5, &c; and 1 and 4 is the same as 2 and 5 or 3 and 6. These chlorine derivatives may be converted into numerous others by replacing the atoms of chlorine by other atoms or groups of atoms ; and so three series of compounds are obtainable, all of which belong to three separate groups. It has been found possible to determine the positions relative to each other of the entering atoms by the following ingenious device. Expressing, for shortness' sake, the structural formula above given by a simple hexagon, and assuming that carbon atoms are situated at the angles of the hexagon, and, where not otherwise indicated, in combination with hydrogen ; but indicating by the symbol CI that an atom of hydrogen has been replaced by one of chlorine, we have the following three formulas for the three dichlorobenzenes :
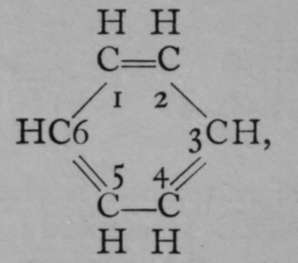
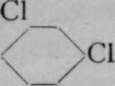
Meta.
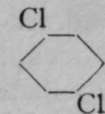
Para.
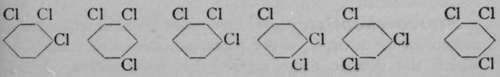
Now, if each of these compounds be separately treated with chlorine, trichlorobenzenes are formed ; these are shown in the lower line ; and it will be noticed that while the first dichlorobenzene (that designated by the prefix "ortho-") can yield two, and only two, trichlorobenzenes, the " meta-" dichlorobenzene may yield three trichlorobenzenes, but the " para-" dichlorobenzene only one ; or in numbers, i 2 may yield 123 and 124 trichlorobenzenes ; 1 3 may yield 1 2 3, 1 3 4, and 135 trichlorobenzenes ; while 1 4 di-chlorobenzenes can yield only 124 trichlorobenzenes ; the last is obviously identical with 1 3 4, or with 1 4 5, or with 146 trichlorobenzene. In this way the actual position of the chlorine atoms, relatively to each other, in the three dichlorobenzenes, was established.
All these formulas, it will be noticed, are written on the assumption that the atoms of the elements lie in a plane. Now this assumption is exceedingly improbable. It is true that a map represents only the length and breadth of a country; not the height of the mountains, unless contour lines be made use of. And yet a map renders great service, for we use it, allowing for this important omission. So these structural formulas may be advantageously employed, so long as we remember that they do not represent all the structure. This fact must have been in the minds of many chemists for more than ten years before J. A. LeBel and J. H. van't Hoff pointed out independently, in 1874, the necessity of employing formulae of three dimensions in order to explain certain cases of isomerism which were inexplicable on the assumption that the elements were distributed on a plane surface, as in the instances already given. This doctrine of the arrangement of the atoms of a molecule in space of three dimensions has been termed Stereo-chemistry.
Continue to:
