Hypophosphites
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Hypophosphites
Hypophospnorous acid, H3PO2, is a monobasic acid ; sodium hypophosphite has the formula Na(H2PO2). This leads to a formula analogous to that given in (11). When heated, too, the acid yields phosphine and phosphoric acid: 2H3PO2 = PH3 + H3PO4. This would lead to the supposition that some of the hydrogen was already in combination with the phosphorus. Its salts also yield phosphine, together with a phosphate and free hydrogen. The acid is prepared by the action of sulphuric acid on the barium salt; that salt is prepared by boiling together yellow phosphorus and caustic baryta : 2P4 + 3Ba(OH)2.Aq + 6H2O - 2PH3 + 3Ba(H2PO2)2.Aq. With sulphuric acid insoluble barium sulphate is formed, while hypophosphorous acid remains in solution. It forms white crystals, melting at 17.4°. The acid has reducing power; with silver nitrate, for example, metallic silver is precipitated and phosphoric acid is formed. With hydrogen iodide phosphorous acid and phosphonium iodide are formed: 3H(H2PO2) + HI = 2H2(HPO3) + PH4I.
Two acids are known belonging to this group of elements ; they have not been tabulated on p. 132, because their structure may be compared with that of hydrazine or liquid phosphine, H2N-NH2 or H2P-PH2, in which two atoms of nitrogen-or of phosphorus-are in direct union with each other. These are phosphatic acid, or, as it is sometimes termed, hypophosphoric acid, 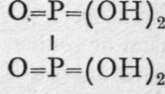 , and hyponitrous acid,
, and hyponitrous acid, ![]() The first of these is produced in small quantity along with ortho-phosphoric and phosphorous acids, when phosphorus is oxidised by exposure to moist air. It is, however, best made from its silver salt, by addition of the equivalent quantity of hydrochloric acid. Silver hypophosphate is produced by dissolving 6 grams of silver in 100 grams of nitric acid diluted with its own weight of water, and adding to the solution, warmed on a waterbath, 8 or 9 grams of phosphorus. As soon as the violent evolution of nitrous gases ceases the liquid is cooled, and silver hypophosphate crystallises out. The acid has no reducing properties, hence it probably contains no hydrogen capable of conversion into hydroxyl by the addition of oxygen. The sodium salt, Na4P2O6, is converted into pyrophosphate by the action of a solution of bromine in water ; the change is evidently due to the addition of oxygen, thus :
The first of these is produced in small quantity along with ortho-phosphoric and phosphorous acids, when phosphorus is oxidised by exposure to moist air. It is, however, best made from its silver salt, by addition of the equivalent quantity of hydrochloric acid. Silver hypophosphate is produced by dissolving 6 grams of silver in 100 grams of nitric acid diluted with its own weight of water, and adding to the solution, warmed on a waterbath, 8 or 9 grams of phosphorus. As soon as the violent evolution of nitrous gases ceases the liquid is cooled, and silver hypophosphate crystallises out. The acid has no reducing properties, hence it probably contains no hydrogen capable of conversion into hydroxyl by the addition of oxygen. The sodium salt, Na4P2O6, is converted into pyrophosphate by the action of a solution of bromine in water ; the change is evidently due to the addition of oxygen, thus : 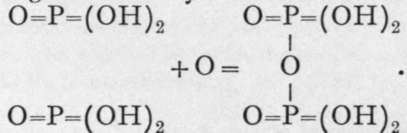 The anhydride of this acid would be
The anhydride of this acid would be ![]() a compound of the formula P2O4 is produced by the incomplete combustion of phosphorus in oxygen; but as it yields orthophosphoric and phosphorous acids on treatment with water, it is in all probability phosphoryl phosphate,
a compound of the formula P2O4 is produced by the incomplete combustion of phosphorus in oxygen; but as it yields orthophosphoric and phosphorous acids on treatment with water, it is in all probability phosphoryl phosphate, 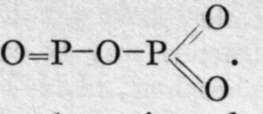
Hyponitrites are produced by the action of sodium amalgam, that is, a solution of sodium in mercury containing about 4 per cent, of the former, on a solution of potassium or sodium nitrite. After the mixture has stood for some days, it is rendered slightly acid with acetic acid, and silver nitrate is added. A yellow precipitate of silver hyponitrite is produced ; other hyponitrites may be prepared from it by the addition of the calculated quantity of the respective chloride. The acid can also be liberated by the addition to a very dilute aqueous solution of the equivalent amount of hydrochloric acid. On warming the solution of the acid, nitrous oxide is evolved ; but nitrous oxide does not unite with water to form the acid.
That the acid has the formula H2N2O0, and not HNO, is shown by its formation from hydroxylamine and nitrous acid. On mixing dilute solutions of hydroxylamine sulphate and sodium nitrite, the hydroxylamine nitrite loses water, thus :
HO-NH2 + 0=N-OH = H2O 4- HO-N=N-OH ; the silver salt is thrown down on addition of silver nitrate.
Continue to:
