Chapter VIII. The Oxy-Acids Of The Halogens
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Chapter VIII. The Oxy-Acids Of The Halogens
The formulae of the acids of the halogens present some analogy with those of the nitrogen group, for, like the latter, the halogens also possess uneven valency. But while the highest valency of elements of the nitrogen group is that of a pentad, chlorine and iodine function as heptads in perchloric and periodic acids. The valency of the halogens is five in chloric bromic, and iodic acids; three in chlorous acid; and one in the hypochlorites, hypobromites, and hypoiodites. According to the hypothesis of electrons, we must suppose that when the halogens function as heptads, all their electrons come into play. These electrons are represented by dashes or " bonds " in the formulae given below. Only the electrons belonging to the iodine are thus expressed; those of the oxygen, silver, hydrogen, and sodium are omitted. They might, however, if it were thought desirable, be introduced, thus: 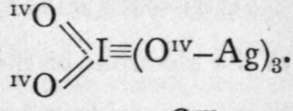
The electrons associated with the oxygen atoms, Oiv, may be regarded as latent. A short table, analogous to that given on p. 132, shows the relation between these compounds:- I(OH)(.(ONa), corresponding to I(OH)7, ortho-periodates ;
0 = I(OAg)5, corresponding to 0 = I(OH)5, para-periodates ;
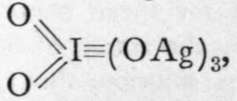 corresponding tc, meso-
corresponding tc, meso-
(middle) periodates ;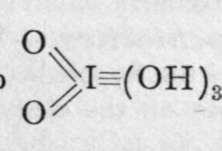
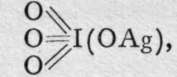 corresponding tometaperiodates.
corresponding tometaperiodates.![]()
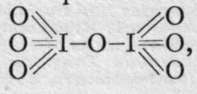 unknown periodic anhydride.
unknown periodic anhydride.
Perchloric acid, O3Cl(OH), corresponding to meta-periodic acid, is the only representative of these among the other members of the halogen group.
T(OH)5, ortho iodic acid; orthobromic and ortho-chloric acids are unknown.
0=I(OH)3, and similar bromine and chlorine acids, are unknown.
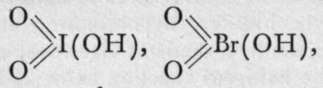 andiodic, bromic, and chloric acids.
andiodic, bromic, and chloric acids.![]()
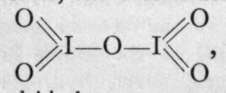 iodic anhydride, is the only anhydride known.
iodic anhydride, is the only anhydride known.
A tri-iodic acid, HI3O8, has been prepared. Here two of the electrons of the halogen atom are latent, thus : 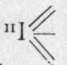
Similar considerations may be applied to the triad and monad compounds of the halogens.
O = Cl-(OH), chlorous acid, is the only representative of triad halide.
I-(ONa), Br-(ONa), and Cl-(ONa), hypoiodite, -chlorite, and -bromite of sodium and of some other metals are fairly stable in solution.
Continue to:
- prev: Hypophosphites
- Table of Contents
- next: Hypochlorites
