General Nature Of The Hydrides
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
General Nature Of The Hydrides
Hydrides of lithium, sodium, potassium, iron, nickel, palladium, and platinum differ from the others in character ; they are solid bodies, decomposed by heat. Graham, indeed, who investigated that of palladium, was struck with the metallic nature of the substance, and was inclined to believe that it might be regarded as an alloy of a metallic form of hydrogen, to which he gave the name "hydrogenium;" and it was for long believed that liquid hydrogen would show the characteristic property of metals, metallic lustre. But this anticipation has not been fulfilled. Liquid hydrogen is a colourless body; and solid hydrogen has a white crystalline appearance, like ice froth. But it must be confessed that hydrogen shows a marked similarity to metals in many of its compounds, as will be frequently seen in the sequel.
The remaining hydrides may be divided into three classes :-Those which react with neither acid nor bases, and which may therefore be described as neutral. To this class belong the hydrides of horon, carbon, silicon, arsenic, and antimony. That of phosphorus nearly falls into the same category, for its compounds with acids are very unstable. The next class-those which react with bases-comprises water and the hydrides of sulphur, selenium, and tellurium. The compounds are termed hydroxides, or, in the case of sulphur, hydrosulphides. These will be considered later, but an instance may be given here :-When lime is moistened with water it is slaked, with formation of calcium hydroxide, thus : CaO + H2O = Ca(OH)2. The hydrides of fluorine, chlorine, bromine, and iodine also belong to this class ; but in their case an exchange takes place, thus: CuO + 2HCl.Aq = CuCl2.Aq + H2O. Hydrazoic acid is capable of similar reactions. Such hydrides, with the exception of water, are generally termed acids. The last group of hydrides, ammonia and hydrazine, and, in one or two isolated cases, hydrogen phosphide, unite with acids, forming salts, thus : NH3 + HC1 = NH4C1 ; PH3 + HI = PH4I. It appears that the presence of water is necessary for at least the first of these combinations ; for if perfectly dry hydrogen chloride is mixed with perfectly dry ammonia, no combination results. It is perhaps allowable to suppose that the presence of moisture leads to ionisation of the hydrogen chloride, and that the ionised atoms are capable of entering into combination, while the non-ionised molecule is without action on the ammonia. These compounds will be treated of under the heading of " salts."
The hydrides of boron, carbon, silicon, phosphorus, arsenic, and antimony are insoluble in water; those of nitrogen, sulphur, selenium, tellurium, and the halogens are soluble. With the exception of certain hydrides of carbon, to be afterwards described, and water, all the rest are gases at atmospheric temperature. The fact that water is a liquid, and not, as might be expected, a gas, requires comment. It is noteworthy that water-gas possesses the density 9, corresponding to the molecular weight 18 ; hence there can be no doubt that in the gaseous state water has the formula H2O. But it is known that compounds of sulphur, which are in formulae, and in many properties analogous to compounds of oxygen, possess higher boiling-points than the corresponding oxygen compounds. For instance, bisulphide of carbon, CS2, boils at 44°, whereas carbon dioxide boils at about -8o°. But water boils at 1000, and, contrary to expectation, its analogue, sulphuretted hydrogen, condenses to a liquid at a temperature much below o°. Now, it has been found by a method depending on the rise of liquids in capillary tubes, that while the molecular weight of most substances in the state of liquid is identical with those which they possess in the gaseous state, the molecular weight of water is considerably too great. The conclusion follows, therefore, that the molecular weight of water should be expressed by a more complex formula than H2O ; possibly by H4O2, or by one even more complex. Gaseous hydrogen fluoride, unlike its congeners, has a higher molecular weight than that expressed by the formula HF ; determination of its density leads to the formula H2F2. These facts are probably to be explained by the view that oxygen may possess a higher valency than 2, and fluorine than i, at relatively low temperatures. It is not unlikely that the structural formula of liquid water is 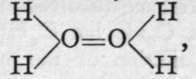 and that of hydrogen fluoride HF=FH, where oxygen acts as a tetrad and fluorine as a triad.
and that of hydrogen fluoride HF=FH, where oxygen acts as a tetrad and fluorine as a triad.
Continue to:
- prev: Acids
- Table of Contents
- next: Hydrocarbons
