Constitution Of Phosphorous Acid
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Constitution Of Phosphorous Acid
Phosphorous trichloride, if treated with a solution of sodium ethoxide, Na( OC2H6), in alcohol, yields tri-ethyl phosphite, (C2H5)3PO3 or P(OC2H5)3, corresponding to P(OH)3. It is a liquid, boiling at 191°. On the other hand, a compound analogous to hydrogen phosphide, PH3, is known, of the formula PH2(C2H5), named di-ethyl phosphine, which, on oxidation, yields a di-basic acid analogous to phosphorous acid, 0=P(C2H5) (OH)2, named ethyl-phosphinic acid, from which the ethyl salt can be prepared, 0=P(C2H5)(OC2H5)2, isomeric with ethyl phosphite. Ethyl phosphite is a derivative of triad phosphorus, whereas di-ethyl ethyl-phosphinate is derived from pentad phosphorus. The anhydride of phosphinic acid, O II 0=PH(OH)2, would be, not P2O3, but H-P=0, like (16). But this substance is not formed on heating phosphorous acid, for it decomposes into phosphoric acid and phosphoretted hydrogen, thus: 4H3PO3 = PH3 + 3HgPO4. One of the varieties of the salts of nitrous acid, however, has a corresponding formula.
Nitrites,-When a nitrate of one of the metals of the alkalies is heated with metallic lead, lead monoxide is formed, and a nitrite, thus : KNO3 + Pb = PbO + KNO9. The nitrite is left as a white fusible salt, easily soluble in water. On acidifying a very dilute solution with sulphuric acid, a dilute solution of nitrous acid is formed ; but on warming it, a somewhat complex action takes place. First, the anhydride is produced: 2HNO9.Aq = H2O.Aq + N2O3 ; next, the anhydride is attacked by the water and decomposed: 3N2O3 + H2O = 2HNO3 + 4NO ; and some of the anhydride volatilises with decomposition into nitric oxide and peroxide : N2O3 = NO + NO2. The nitrites are white, easily soluble crystalline salts; those of lead, Pb(NO2)2, and silver, AgNO2, are sparingly soluble. All are decomposed by the stronger acids ; for nitrous acid is a weak acid, and, moreover, it is easily decomposed, as has been pointed out. None of these changes throws any light on the constitution of nitrous acid, however. To gain this knowledge it is necessary to study the alkyl salts ; for example, ethyl nitrite.
Constitution of the Nitrites, - Ethyl nitrite, made by distilling together a mixture of sodium nitrite, sulphuric acid, and alcohol, is a volatile colourless liquid with a fragrant odour. On boiling it with a solution of sodium hydroxide it is hydrolysed, the ethyl group being again replaced by the metal sodium, thus : 0=N-0(C2H5) + NaOH.Aq = 0=N-ONa.Aq + C2H5OH. And if ethyl nitrite be placed in a flask along with tin and hydrochloric acid-in other words, exposed to the action of nascent hydrogen-the products are ammonia (with some hydroxylamine) and alcohol: 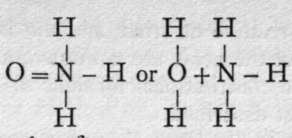 +
+
HO(C2H5). Sodium nitrite therefore appears to possess the formula 0=N-ONa. But silver nitrite, heated in a sealed tube with ethyl iodide, yields a compound of the same composition as, but not identical with, ethyl nitrite, and the formula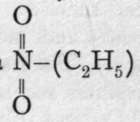 is ascribed to it; for, on heating with caustic soda, it is not hydrolysed, but one of the atoms of hydrogen of the ethvl armm is replaced by the element sodium, giving
is ascribed to it; for, on heating with caustic soda, it is not hydrolysed, but one of the atoms of hydrogen of the ethvl armm is replaced by the element sodium, giving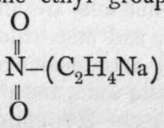 ; and further, with nascent hydrogen, the two atoms of oxygen are removed and replaced by hydrogen, yielding ethylamine, a compound analogous to ammonia,
; and further, with nascent hydrogen, the two atoms of oxygen are removed and replaced by hydrogen, yielding ethylamine, a compound analogous to ammonia,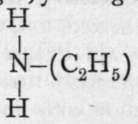 ; this shows that the ethyl group in the compound, which is named nitroethane, is in direct union with the nitrogen atom. It appears, then, that silver nitrite has the formula
; this shows that the ethyl group in the compound, which is named nitroethane, is in direct union with the nitrogen atom. It appears, then, that silver nitrite has the formula ![]() , and not
, and not
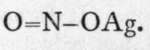 It also follows that two nitrous acids must exist,
It also follows that two nitrous acids must exist,![]() and,
and, ![]() the former (13) a derivative of triad, and the latter (16) of pentad nitrogen. But the acids are unknown, and it is only possible to guess the constitutional formulae of the salts through the reactions just described.
the former (13) a derivative of triad, and the latter (16) of pentad nitrogen. But the acids are unknown, and it is only possible to guess the constitutional formulae of the salts through the reactions just described.
Continue to:
- prev: Phosphorous Acid
- Table of Contents
- next: Arsenites
