Destructive Distillation Synthesis
Description
This section is from the book "Rubber And Rubber Planting", by R. H. Lock. Also available from Amazon: Rubber And Rubber Planting.
Destructive Distillation Synthesis
The destructive distillation of india-rubber gives rise to isoprene CBH8, and to other bodies of the formula (C5H8)». Isoprene is also the most important step in the synthesis of rubber, for which a number of commercial processes have recently been patented. Tilden, many years ago, obtained true rubber from isoprene which had been prepared by passing the vapour of turpentine through heated tubes. The rubber appeared spontaneously in the isoprene which had been kept for some years in a closed bottle, and was also produced more rapidly in small quantities by the action of hydrochloric acid. The last named method of preparation confirmed a previous discovery by Bouchardet.
The production of rubber from isoprene is a process of polymerisation, that is to say the union of a number of molecules each possessing the same empyrical formula, i.e. containing the same relative number of carbon and hydrogen atoms. Analysis has shown that rubber contains carbon and hydrogen in the same proportions as exist in isoprene and turpentine. A study of the compounds of rubber, particularly those with ozone, has led Harries to represent the polymerisation of isoprene in the following manner:
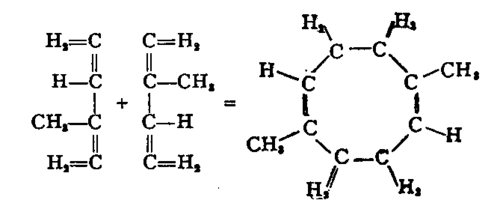
The actual molecule of rubber is probably a polymer of the substance whose constitution is represented above, and may consist of a number of similar eight-ring molecules linked together. The empyrical formula of rubber is thus C10H16, which is identical with those of turpentine and gutta-percha. The molecule of the latter is probably even more complex than that of rubber, but may possibly be a higher polymer of the same series. It should be observed that the account here given of the constitution of the rubber molecule only represents one of the most recent theories. The problem of the structure of this molecule cannot yet be regarded as finally settled.
In some of the modern processes which have been proposed for the manufacture of synthetic rubber on a commercial scale, isoprene is prepared from fusel oil, obtained from starch by special methods of fermentation ; and polymerisation is effected by the action of metallic sodium. Various other substances have also been synthesised, which, though differing in composition, appear to possess a similar molecular structure to india-rubber. The physical properties of some of these bodies are closely similar to those of rubber, and their use has been proposed commercially for similar purposes. So far, none of these substances have been placed upon the market in appreciable quantities, and the reports of the cheap production of synthetic rubber have not carried with them a fall in the value of plantation shares.
Continue to:
