Tautomerism
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Tautomerism
One more kind of isomerism remains to be mentioned ; a body which is said to be tautomeric appears to show a different constitution, according to the reagent with which it is treated. One of the earliest instances observed of a tautomeric compound is aceto-acetate of ethvl. Its formula is :as shown by its reaction with caustic potash, when acetone, CH3ŚCOŚCH3, is formed, the scission occurring at the dotted line ; but the tautomeric formula 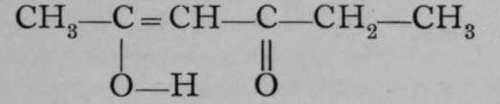 may also be ascribed to it, for it can be shown to contain a hydroxyl group by the action of diethylamine, giving
may also be ascribed to it, for it can be shown to contain a hydroxyl group by the action of diethylamine, giving 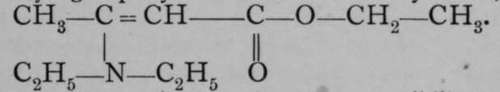 .
.
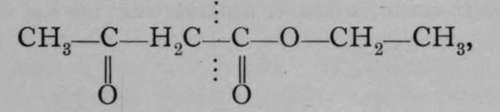
Other reactions point to the same possibility of rearrangement. Examples of tautomerism are not unknown among compounds of elements other than carbon ; it is probable that two sulphurous acids are ramble of existence, one possessing the formula 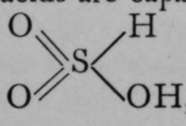 and the other.Silver sulphite appears to be a derivative of the
and the other.Silver sulphite appears to be a derivative of the 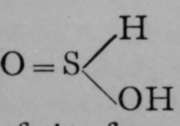 first, and sodium sulphite of the second of these forms ; and it is probable that the particular form taken depends on the reagent which is presented to the acid.
first, and sodium sulphite of the second of these forms ; and it is probable that the particular form taken depends on the reagent which is presented to the acid.
Although the application of geometrical formulae has proved useful in exhibiting certain cases of isomerism such as have been considered, it is not to be supposed that formulae to which grouping in space of three dimensions is-not usually applied do not also require three-dimensional space. Their use is not common, merely because the spatial relations are sufficiently evident without involving this conception. Most of us are content with a picture as a sufficient memento of our friends ; but if we wish a fuller presentment, a bust or a statue will give it.
Continue to:
