Endothermic Combination
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Endothermic Combination
This body is fearfully explosive, for its formation is attended by great absorption of heat ; but during its formation the reagents do not grow cold ; for the formation of ammonium chloride is a highly exothermic reaction, and the amount of heat evolved by its formation is greater than that of the equivalent amount of chloride of nitrogen ; hence the change as a whole is accompanied by evolution of heat. It is thus that endothermic compounds are usually formed : by virtue of a simultaneous action in which heat is evolved. The slightest shock causes the decomposition of such endothermic bodies ; if one single molecule is decomposed, it evolves heat and brings about the decomposition of its neighbours ; and as all the molecules are in close proximity to each other, and as the products, nitrogen and chlorine, are both gases, and are, moreover, much raised in temperature by being set free, the decomposition is accompanied by sudden and enormous expansion. Nitrogen iodide, prepared by adding a solution of iodine to aqueous ammonia, is a black solid of the formula NI3.NH3 ; it is also explosive.
The fluorides of boron and silicon are both produced by the action of a strong solution of hydrofluoric acid on the oxides ; but it is necessary to have some agent present to withdraw water, such as concentrated sulphuric acid. These compounds are both gaseous. Their formation is shown by the equations: B2O3 + 6HF = 2BF3 + 3H2O ; SiO2 + 4HF = SiF4 + 2H2O. If the water is not withdrawn, combination ensues between the fluoride and hydrogen fluoride, with formation of HBF4 or H2SiF6, named respectively hydroborofluoric, and hydrosilicifluoric acids thus: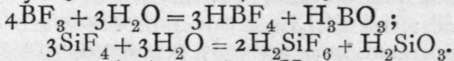
These compounds ionise into H-ions, and the complex ions - BF4 and =SiF6 ; and many salts are known in which metals replace the hydrogen. They are similar in kind to potassium aurichloride.
The other halides of boron and silicon, and also of phosphorus, sulphur, selenium, tellurium, and iodine, react at once with water, forming hydrogen halide and an acid. The equations are as follows :-
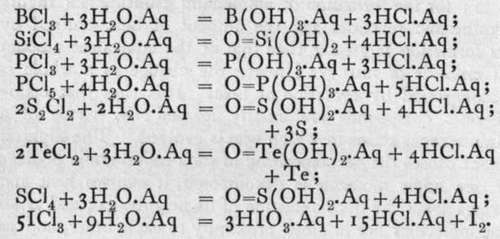
It is to be noticed that where a hydroxy-compound corresponding to the halide is capable of existence, it is formed; if not, excess of the element is set free. Hence none of these halides can be prepared by acting on the hydroxide with a halogen acid; they are all made either by the direct action of the halogen on the element, or by what comes to the same thing, the action of the halogen on a strongly heated mixture of the oxide of the element with carbon. Boron, silicon, and phosphorous chlorides are volatile liquids; they fume in the air owing to their action on the water-vapour. S2C12 is a yellow liquid ; when saturated with chlorine at a low temperature, SC12 and SC14 are successively formed; but on rise of temperature they dissociate into the lower chloride. ICl is a black solid, converted by excess of chlorine at a low temperature into IC13, a yellow solid, which easily dissociates into ICl and Cl2; and PC15 is a pale yellow solid, volatile at a high temperature in a perfectly dry atmosphere without dissociation, but resolved by the least trace of moisture into PC13 and Cl2.
Continue to:
