Conductivity Of Electrolytes
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Conductivity Of Electrolytes
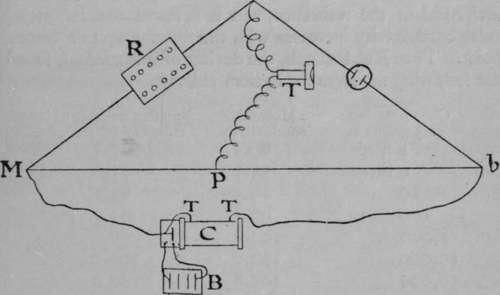
To measure the conductivity of a solution, a "gram-molecular-weight," i.e. the molecular weight of the salt taken in grams, is dissolved in a litre of water. A small quantity of this solution is placed in a small beaker immersed in a large tank of water, so that the temperature may not vary ; for the conductivity increases with rise of temperature, owing to the smaller resistance offered by hot water to the passage of the ions, than by cold. Two circular platinum plates, the surfaces of which have been roughened by having platinum deposited on them, are immersed in the solution, so that one lies at the bottom of the beaker, while the other is one centimeter distant from it, higher up in the liquid. The wire connected with the lower plate is protected by a glass tube, in order that the current may pass only between the plates. To measure the conductivity of this solution an arrangement termed a " Wheatstone's bridge " is employed, the construction of which will be understood from the annexed diagram. B is a battery, actuating a small toy coil, C, from the secondary termmais of which T T wires proceed to the measuring-bridge Mb, which consists of a straight piece of nickel-silver wire stretched along a scale. From one end of the bridge, b, the current traverses the solution ; from the other, M, the current passes through a resistance-box, R, containing bobbins of wire of known resistance, the number and resistance of which can be varied at will until the resistance is nearly equal to that of the solution. If they are exactly equal, and if the pointer P is exactly in the middle of the bridge-wire Mb, then no sound can be heard in the telephone T. The resistance of the solution can then be read from the box. If, as generally happens, the resistance of the solution is not equal to that in the box, it is necessary to move the pointer P until the resistances are equal. Having thus ascertained the resistance of the solution, a portion is diluted with water, so that its strength is exactly half of the former, and the resistance is again determined. Successive dilutions in which the volume of the solution is doubled, and again doubled, are made, and in this manner the resistance due to an equal number of molecules in each case is calculated. The conductivity is the reciprocal of the resistance, and it is found that the molecular conductivity increases with the dilution up to a certain point. Thus Kohlrausch, the deviser of this method, found the following numbers for sodium chloride:
Concentration : | Molecular | Relative number of ions |
58.5 grams in | conductivity. | per 100 molecules of salt. |
i litre | 69.5 | 67.5 |
2 litres | 75-7 | 73-6 |
10 | 86.5 | 84.1 |
IOO | 96.2 | 93-5 |
1,000 | 100.8 | 98.0 |
10,000 ,, | 102.9 | 100.0 |
50,000 | 102.8 | 100.0 |
It is evident that 58.5 grams of salt dissolved in 10,000 litres of water give a maximum conductivity, for the dilution to 50,000 litres is attended by no further increase. That the altered velocity is not influenced by the frictional resistance of the water is obvious, for the solution of 58.5 grams of salt in 10 litres of water does not differ much from pure water in this respect. The increase in conductivity must accordingly be attributed to an increase in the number of ions at the expense of the molecules ; and, as a dilution in 50,000 litres of water produces no greater conductivity than in 10,000, it must be concluded that complete ionisation has taken place. The figures in the last column are obtained by simple proportion, thus :As 102.9 : 69-5 ::100: 67.5.
The extent of ionisation calculated from the conductivities of salt solutions agrees well in the main with that calculated from the depressions of freezing-point.
Continue to:
