Carbamates
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Carbamates
Ammonium carbamate, known by the familiar name of " smelling salts," is formed by mixing ammonia and carbon dioxide gases: CO2 + 2NH3 = H2N - CO - ONH4. It is a white crystalline compound, soluble in water and smelling of ammonia. Its solution, when fresh, contains the compound of which the formula is given above; but after standing, it is converted by absorption of water into ammonium carbonate. This has been ascertained by treating the freshly prepared solution with sodium hypochlorite, when only half the nitrogen which the substance contains is evolved :
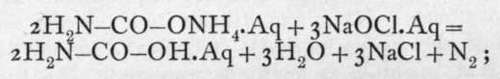 on the other hand, with a hypobromite, all the nitrogen is evolved :
on the other hand, with a hypobromite, all the nitrogen is evolved :
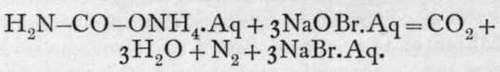
Now, ammonium salts yield up their nitrogen when mixed with a solution of a hypochlorite; hence it is concluded that the compound contained in a fresh solution is ammonium carbamate. But on standing, the solution changes, and after some time it yields all its nitrogen on treatment with hypochlorite ; hence the assumption of the elements of water and a change into ammonium carbonate may be inferred: H2N-CO-ONH4.Aq + H2O = H4N-O-CO-ONH4. Aq. But ammonium carbamate may conceivably possess the formula HO-C(NH)-ONH4; and it may be that it is the =NH group which resists attack. This last supposition is confirmed by the behaviour of urea with hypochlorite; for with it, too, only half the nitrogen is evolved.
Continue to:
- prev: Amines
- Table of Contents
- next: Carbamide
