Amides Of Acids Of Phosphorus
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Amides Of Acids Of Phosphorus
Many compounds analogous to urea are known, where the hydroxyl groups of acids are replaced by amido-groups, -NH9. By the action of ammonia gas on phosphorus oxychloride ortho-phosphamide is formed : 0=PC13 + 3HNH2 = 0=P(NH2)3 + 3HCI. The ammonium chloride formed by the combination of the hydrochloric acid with excess of ammonia is removed by washing, and an insoluble white powder remains. When phosphamide is heated, ammonia is lost, and phosphoryl-amide-imide (the group =NH is termed the " imido-group"), HN=PO(NH2), and at a higher temperature, ![]() or phosphoryl nitride are left. They are also white insoluble powders. By analogy with carbamic acid and urea, there should exist compounds in which both hydroxyl and the amido-group are present. Some such compounds are known. Thiophosphamic acid, S=P(NH2)(OH)2, is the product of the action of ammonia on thiophosphoryl chloride; and phosphoric anhydride, when dry ammonia gas is passed over it, yields phosphimic acid, thus:
or phosphoryl nitride are left. They are also white insoluble powders. By analogy with carbamic acid and urea, there should exist compounds in which both hydroxyl and the amido-group are present. Some such compounds are known. Thiophosphamic acid, S=P(NH2)(OH)2, is the product of the action of ammonia on thiophosphoryl chloride; and phosphoric anhydride, when dry ammonia gas is passed over it, yields phosphimic acid, thus: 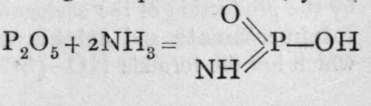
+ H2O. It is analogous to metaphosphoric acid,
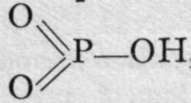 , and forms crystalline salts. Pyrophosphamic acids are also known. The addition of phos-phoryl chloride to a cold saturated solution of ammonia results in the formation of pyrophospho- diamic acid
, and forms crystalline salts. Pyrophosphamic acids are also known. The addition of phos-phoryl chloride to a cold saturated solution of ammonia results in the formation of pyrophospho- diamic acid
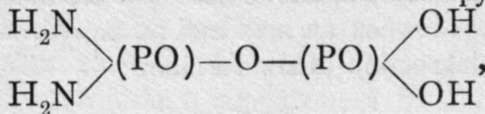 i analogous to pyrophosphoric acid,
i analogous to pyrophosphoric acid, 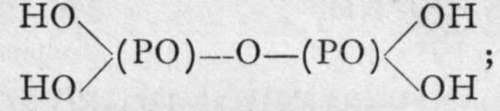 and on heating the solution of this body, one hydroxyl group replaces one amido-group, yielding pyrophosphamic acid,
and on heating the solution of this body, one hydroxyl group replaces one amido-group, yielding pyrophosphamic acid,
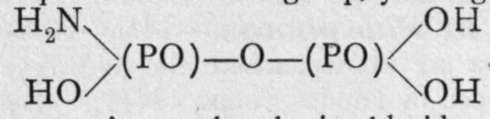 Lastly, the action of ammonia on phosphoric chloride gives a compound named phospham,
Lastly, the action of ammonia on phosphoric chloride gives a compound named phospham, ![]() a species of anhydride, but produced by loss of ammonia, not of water, from the unknown compound P(NH2)6.
a species of anhydride, but produced by loss of ammonia, not of water, from the unknown compound P(NH2)6.
Analogues of phosphorous acid are less well known; if ammonia be passed over phosphorous chloride, a white mass is formed, which has not been separated from ammonium chloride, but which is supposed to possess the formula P(NH2)3; it may be named phosphorosamide.
Continue to:
