Action Of Nitric Acid On Metals
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Action Of Nitric Acid On Metals
The product of the action of nitric acid on metals varies according to the metal acted on, the concentration of the acid, and the temperature. The acid in aqueous solution is more or less ionised, the ions being H and -NOg. If a metal of which the ions are highly electropositive, that is, which readily parts with electrons, is presented to these ions of nitric acid the hydrogen ions receive electrons from the non-ionised metal, which metal enters into solution as ions, while hydrogen is evolved. This is the case when nitric acid acts on magnesium, and theoretically also on aluminium, manganese, zinc, cadmium, iron, cobalt, and nickel, for all these metals in the ionic state have higher electro-affinity, that is, they part with electrons more readily than hydrogen, and that in the order given. It may be termed the normal action of acids on metals, and represented thus:
![]() But along with this action others take place in which the nitric ion is " reduced" or deprived of oxygen. Some examples of this will now be given.
But along with this action others take place in which the nitric ion is " reduced" or deprived of oxygen. Some examples of this will now be given.
When silver is attacked by nitric acid, nitric peroxide, NO2, is produced, and partly evolved as eas. The reacting substances are Ag, and ![]() and
and ![]() one of the
one of the
-NO3 groups loses oxygen, being converted into electrically neutral NO2 and an ion of oxygen, =0, which combines with the two hydrogen ions, forming water, non-ionised, H2O. But this leaves a nitrate group combined with an electron without a corresponding partner which has lost an electron; moreover, the charge of the decomposed nitrate group is still available. An atom of silver, therefore, goes into solution as an ion, and transfers its electron to the NO3 group. With less concentrated acid the nitrate ion parts with two atoms of oxygen, requiring three electrons, in addition to the one originally attached to the group -NO3; to effect this three electrons must leave three atoms of silver, which then go into solution as ions, hence the change is :
![]() the balance of electric charge has, nevertheless, not been disturbed, although four electrons have ceased to be transferable. With metals yielding kations of higher potential, the reduction of the nitrate ion goes still farther; nitrous oxide, N2O, nitrogen, and even ammonia may be produced, in relative amounts depending on the metal, on the concentration, and on the temperature. It may be taken that the lower the temperature, the less the concentration, and the higher the metal stands in the electro-negative series, the greater the reduction. The equations are:
the balance of electric charge has, nevertheless, not been disturbed, although four electrons have ceased to be transferable. With metals yielding kations of higher potential, the reduction of the nitrate ion goes still farther; nitrous oxide, N2O, nitrogen, and even ammonia may be produced, in relative amounts depending on the metal, on the concentration, and on the temperature. It may be taken that the lower the temperature, the less the concentration, and the higher the metal stands in the electro-negative series, the greater the reduction. The equations are:
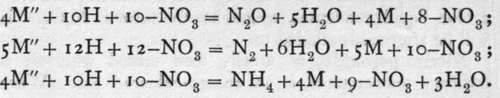
All these changes may proceed simultaneously ; but copper and moderately strong nitric acid yields fairly pure nitric oxide ; if more concentrated acid be employed, a mixture of varying proportions of nitric oxide and peroxide are evolved; while by using zinc or magnesium and very dilute acid, nitrous oxide, nitrogen, hydrogen, and ammonium nitrate are the main products.
Continue to:
