Aldehydes
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Aldehydes
The alcohols, if oxidised by boiling them with chromic acid, yield a class of bodies analogous to the oxides, termed aldehydes: CHg-CH2-OH + O = (CH3 - CH)"0 + H2O. It will be noticed that ethane, CH3 - CH3, has lost two hydrogen atoms, and that the residue, CH3 - CH, is now a dyad group, capable of combination with an atom of dyad oxygen. The aldehydes are volatile liquids, with strong odour, and those containing few atoms of carbon are miscible with water. They form easily decomposable compounds with water, which are di-hydroxides; e.g. ordinary aldehyde forms 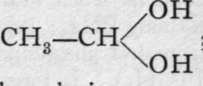 ; they are called aldehydrols. When brought into contact with solutions from which hydrogen is being evolved, the aldehvdrols lose oxvgen. and are converted into alcohols:
; they are called aldehydrols. When brought into contact with solutions from which hydrogen is being evolved, the aldehvdrols lose oxvgen. and are converted into alcohols:
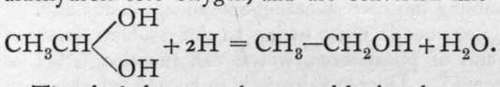
The alcohols cannot be termed basic substances ; still, it is evident that they show analogy with the true bases in many respects.
Continue to:
- prev: Alcohols
- Table of Contents
- next: Amines And Phosphines
