Alcohols
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Alcohols
The hydroxides of the hydrocarbon radicles are, as mentioned on p. 67, termed alcohols.1 Of these there are very many, but a few only will be chosen to serve as examples: methyl alcohol, CH3OH, ethyl alcohol, CH3-CH2OH, as types of monohydric alcohols, which may be taken as the analogues of the hydroxides of the monad metals; glycol, 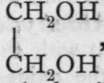 , a dihydric alcohol, may be likened to barium hydroxide,
, a dihydric alcohol, may be likened to barium hydroxide,
1 A special class of such hydroxides derived from benzene, C6H6, are termed phenols. 11 Carbolic acid," C6H5OH, is the best known of these.
Ba(OH)2; and glycerine (glycerol),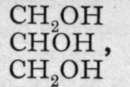 is a trihydric alcohol, as aluminium hydroxide is a trihydroxide. These substances differ from the hydroxides, however, by their being non-electrolytes, and therefore non-ionised. Or perhaps it is more correct to say that their conductivity is of the same order of magnitude, but less in value, than that of pure water. The corresponding halides, for example, CH3C1, C2H4C12, and C3H5C13, are also regarded as non-ionised ; they are practically insoluble in water. Nevertheless, methyl chloride has been transformed into methyl alcohol by heating with water to a high temperature in a sealed tube under pressure-CH3C1 + HOH = CH3OH + HC1; and the others, but preferably the bromides, may be similarly changed into hydroxides by heating with silver hvdroxide. or with silver oxide and water :
is a trihydric alcohol, as aluminium hydroxide is a trihydroxide. These substances differ from the hydroxides, however, by their being non-electrolytes, and therefore non-ionised. Or perhaps it is more correct to say that their conductivity is of the same order of magnitude, but less in value, than that of pure water. The corresponding halides, for example, CH3C1, C2H4C12, and C3H5C13, are also regarded as non-ionised ; they are practically insoluble in water. Nevertheless, methyl chloride has been transformed into methyl alcohol by heating with water to a high temperature in a sealed tube under pressure-CH3C1 + HOH = CH3OH + HC1; and the others, but preferably the bromides, may be similarly changed into hydroxides by heating with silver hvdroxide. or with silver oxide and water :
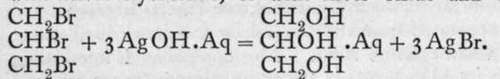 Is it possible that at a higher temperature the ionisation is sufficient (though it must be exceedingly small) to produce the interaction ?
Is it possible that at a higher temperature the ionisation is sufficient (though it must be exceedingly small) to produce the interaction ?
The metals sodium and potassium dissolve in the alcohols, with evolution of hydrogen, forming compounds somewhat analogous to the hydroxides ; instead of hydrogen, however, they contain a hydrocarbon group : sodium methoxide, for example, has the formula Na(OCH3). Such substances are white solids, like caustic soda.
Continue to:
