Phosphates
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Phosphates
The source of phosphoric acid and the phosphates is chiefly calcium phosphate, Ca3(PO4)2, a mineral known as phosphorite, and A1PO4, aluminium phosphate, or gibbsite. Phosphoric acid is produced from phosphorite by heating it with dilute sulphuric acid ; sparingly soluble calcium phosphate is formed, while ortho-phosphoric acid goes into solution. The solution, on evaporation, deposits white crystals of H3PO4 ; the residual liquor deposits crystals of H3PO4.H2O ; commercial or " glacial phosphoric acid " is a mixture of both kinds. Its solution contains many hydrogen ions, and it is therefore a strong acid. But, inasmuch as phosphoric acid can ionise in three ways, into
3H and =PO4, 2H and =HPO4, H and -H2PO4, there are three kinds of anions. The first of these, =PO4, are present in very small relative amount; the second and third, =HPO4 and -H2PO4, are relatively much more numerous. This is generally the case with poly basic acids; for instance, sulphuric acid contains the anions - HSO4 and =SO4; the former are the more numerous in this case. There is a state of balance between the quantities of these ions present in any solution; and if, for example, kations of calcium or lead or silver be added to a solution of phosphoric acid, or to one of hydrogen di-sodium phosphate, Na2HPO4, the =PO4 ions present enter into combination with the kations, forming
Ca8(PO4)2) Pb8(PO4)2) or Ag3PO4; the =PO4 ions are increased at the expense of the =HPO4 and -H2PO4 ions, and the solution becomes more acid. On adding an alkali, e.g. caustic soda, to a solution of phosphoric acid, neutrality occurs when the salt Na2HPO4 has been reached; the ions are then mainly 2Na and =HPO4. On adding more soda, the solution becomes alkaline, indicating the presence of free -OH ions; and it is only on concentration that these -OH ions combine with the few H ions of the ionised Na2|=HPO4, forming non-ionised water, and " tri-basic " sodium phosphate, Na3PO4, is left as a residue. Similar remarks apply to the ortho-arsenates. The ortho-vanadates are hydrolysed by water into pyro- and meta-vanadates.
The chief orthophosphates are: Na2HP04.12H2O, obtained by neutralising phosphoric acid with sodium carbonate ; HNa(NH4)PO4.4H2O, named " microcosmic salt"; the human organism used to be known as the " microcosm," and this salt crystallises out of concentrated urine; Ca3(PO4)2,prepared by precipitation,and found native as phosphorite ;, a widely spread mineral termed apatite ; 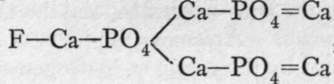 (NH4)MgPO4.6H2O, a white precipitate produced by adding ammonium and magnesium ions to those of a soluble phosphate:
(NH4)MgPO4.6H2O, a white precipitate produced by adding ammonium and magnesium ions to those of a soluble phosphate:
![]()
It is the distinguishing test generally employed to recognise the presence of magnesia, and serves at the same time to distinguish phosphoric acid; arsenates give a precisely similar precipitate. The precipitate is nearly insoluble in ammoniacal water, and it may be filtered off and washed with water containing ammonia with very little loss. Like almost all phosphates, it is soluble in water containing hydrogen ions; and by the addition of ammonium hydroxide their number is greatly diminished. On ignition, it yields magnesium pyrophosphate, Mg2P2O7, thus:
![]()
Continue to:
- prev: Nitrates
- Table of Contents
- next: Arsenates
