Oxidation By Means Of Nitric Acid
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Oxidation By Means Of Nitric Acid
Action of the same nature occurs when an element capable of changing its valency, i.e. the number of electrons associated with its ionised atom, is treated in the ionic condition with nitric acid. For example, the ferrous ion, ( = )Fe, on treatment with nitric acid at ioo° becomes ferric, (=)Fe, while nitric oxide is evolved:
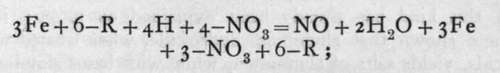
-R being any monovalent anion. Such operations are usually spoken of as "oxidations in the wet way."
Nitric oxide is a colourless gas, very sparingly soluble in water ; on bringing it into contact with oxygen, unless moisture is absolutely excluded, union takes place to form nitric peroxide, NO2, along with a trace of N2O3, nitrous anhydride. On sufficiently cooling nitric oxide it condenses to a colourless liquid, and at a still lower temperature it forms a white solid.
Nitrous anhydride, strictly speaking, belongs to the class of acid-forming oxides ; its formula is N2O3. When nitric oxide and nitric peroxide are brought together, only a minute quantity of N2O3 is formed; that is, because on converting it into the gaseous state it decomposes almost completely into these products. On cooling such a mixture, however, a blue liquid condenses, which has the formula N9Og. It will be afterwards alluded to.
Nitric peroxide, as usually seen mixed with air at ordinary temperatures, is an orange-coloured gas. When pure it condenses to an orange-red liquid, boiling at 22°; it freezes at -io° to a colourless solid. The liquid has a molecular weight corresponding to the formula N2O4, and the gas, at temperatures not much exceeding the boiling-point, consists mainly of the same substance. But as the temperature rises the colour grows darker, until, at 140% it forms a blackish-red gas, consisting wholly of NO£. With progressive increase of temperature NO2 dissociates in its turn into NO and O2, and at 6oo° the change is complete. As temperature falls the action is reversed.
Continue to:
