Compounds Containing Less Oxygen
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Compounds Containing Less Oxygen
The elements of the nitrogen group are characterised by their possessing more than one valency. They are also, in most cases, capable of forming compounds with hydrogen. These two characteristics, taken together, lead to the possibility of their forming a number of isomeric compounds, i.e. compounds having an identical composition, but being, at the same time, different chemical individuals. Some such compounds are known, at least in their derivatives. The conception will be clearer after inspection of the following table :-
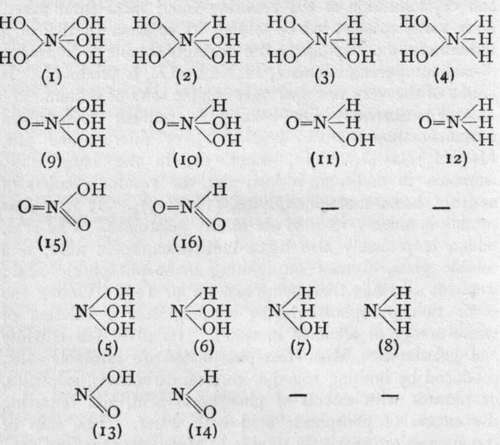
For convenience' sake, the compounds have been written as derivatives of nitrogen, but the type is followed by other elements of the group.
(i) is the true "ortho" acid, unknown in all cases, (9) is its first anhydride, known in " orthophosphoric" acid and in " orthoarsenic" acid. is nitric acid, and corresponds to mono-metaphosphoric acid, metavana-dic acid, the metarsenates, and the metantimonates. (2) and (10) are unknown bodies, but (10) corresponds to phosphorous acid, and (16) to the nitrites, (3) is also unknown, but (n) is represented by hypophosphorous acid and the hypophosphites. (4) is unknown. (12), however, may possibly be the formula of hydroxylamine; its name, in that case, should be " oxy-ammonia." In all these compounds the element is a pentad. The other compounds contain triad element. ( 5) probably represents the formula? of the arsenites ; ( 13 ) is an alternative formula for nitrites. (6) and (14) are unknown. (7) is an alternative formula for hydroxylamine.
Continue to:
- prev: Meta- Salts
- Table of Contents
- next: Phosphorous Acid
