Amines
Description
This section is from the book "Modern Chemistry", by William Ramsay. Also available from Amazon: Modern Chemistry: Theoretical and Modern Chemistry (Volume 2).
Amines
Substituted ammonia, in which one atom of hydrogen is replaced by an element, is the analogue of the hydroxides. Such bodies are termed amines or amides. Sodamine, NaNH2, is easily prepared by passing a current of ammonia, dried by passing it through a tower filled with soda-lime, through an iron U-tube containing sodium, and heated to about 300°. The gas is rapidly absorbed, while hydrogen is evolved : 2NH3 + 2Na = 2NaNH2 + H2. When the sodium has been all converted into sodamine, the tube is emptied by pouring out its contents. Sodamine is a white brittle substance, with crystalline fracture, not unlike caustic soda, melting at about ioo°. So long as it is kept dry it is quite permanent, but with moisture it at once reacts, forming ammonia and caustic soda : NaNH2 + HOH = NaOH + NH3. Similar compounds can be made with lithium, potassium, rubidium, and probably caesium. The corresponding compound of zinc, Zn(NH2)2, is a white powder, insoluble in ether, formed along with ethane or methane by the action of ammonia on zinc methide or ethide : Zn(CH3)2 + 2NH3 = Zn(NH2)2 + 2CH4.
Guanidine
An attempt to produce the amine of carbon, C(NH2)4, by the action of ammonia on such a body as carbon tetrachloride or ethyl orthoformate, C(OC2H5)4, according to the equations CC144-4NH3 = C(NH2)4 + 4HC1, or C(OC2H6)4 + 4NH8 = C(NH2)4 + 4HOC2H5, fails, owing to loss of ammonia. For just as orthocarbonic acid, C(OH)4, loses water, yielding ordinary carbonic acid, so carbon tetramine loses ammonia ; the product is named guanidine, and has the formula HN = C(NH2)2; its analogy with 0=C(OH)2is easily seen. Guanidine is a white crystalline substance, which, like ammonia, unites with acids to form salts.
On comparing the formulae of carbonic acid and guanidine, it is evident that several intermediate compounds should be capable of existence. The series is:-
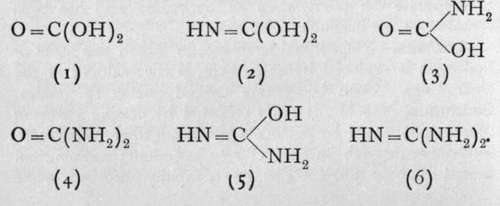
Of these, the best known are the ammonium salt of (3), which is termed carbamic acid, and (4), the important compound urea or carbamide.
Continue to:
- prev: Nitrides
- Table of Contents
- next: Carbamates
